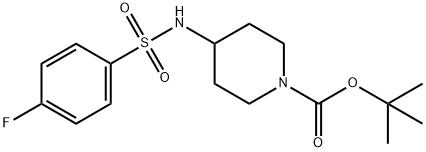
4-(4-Fluoro-benzenesulfonylaMino)-piperidine-1-carboxylic acid tert-butyl ester synthesis
- Product Name:4-(4-Fluoro-benzenesulfonylaMino)-piperidine-1-carboxylic acid tert-butyl ester
- CAS Number:913634-49-8
- Molecular formula:C16H23FN2O4S
- Molecular Weight:358.43
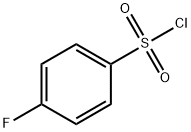
349-88-2
301 suppliers
$6.00/5g
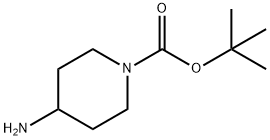
87120-72-7
20 suppliers
$6.00/1g

913634-49-8
16 suppliers
$235.00/250mg
Yield:913634-49-8 89%
Reaction Conditions:
with triethylamine in water;acetonitrile at 20; for 5 h;
Steps:
III.1
To a solution of compound I-c (10 g, 49.93 mmol) in acetone/distilled water (4/1,250 mL) was added compound m-a (R =R =R =R =H, R =F, 9.72 g, 49.93 mmol). A solution of triethylamine (6.96 mL, 49.93 mmol) in distilled water was added and the reaction mixture allowed to stir at room temperature for 5 hours. The solvent was removed in vacuo and the residue dissolved in ethyl acetate. The organic layer was separated and the aqueous phase extracted with ethyl acetate. The combined organic extracts were dried over MgSO 4 , filtered and the solvents removed in vacuo to give a crude product. The crude product was purified by column chromatography to give the title compound as a white solid (16 g, 89%). [136] 1H-NMR(CDCl3): δ 7.75-7.81(m, 2H), 7.08-7.19 (m, 2H), 4.09-4.22 (m, 2H),3.02-3.21 (m, IH), 2.85-2.95 (m, 2H), 1.98-2.04 (m, 2H), 1.47 (s, 9H), 1.41-1.45 (m, 2H).
References:
WO2006/115353,2006,A1 Location in patent:Page/Page column 14
402-46-0
178 suppliers
$11.00/1g

301673-14-3
205 suppliers
$10.00/1g

913634-49-8
16 suppliers
$235.00/250mg

79099-07-3
543 suppliers
$5.00/5g

913634-49-8
16 suppliers
$235.00/250mg