
Methyl 7-Methylpyrazolo[1,5-a]pyriMidine-5-carboxylate synthesis
- Product Name:Methyl 7-Methylpyrazolo[1,5-a]pyriMidine-5-carboxylate
- CAS Number:916211-74-0
- Molecular formula:C9H9N3O2
- Molecular Weight:191.19

1225387-53-0
57 suppliers
inquiry
20577-61-1
180 suppliers
$14.00/1g
![Methyl 5-Methylpyrazolo[1,5-a]pyriMidine-7-carboxylate](/CAS/20150408/GIF/916211-75-1.gif)
916211-75-1
9 suppliers
$1629.00/1g
![Methyl 7-Methylpyrazolo[1,5-a]pyriMidine-5-carboxylate](/CAS/20150408/GIF/916211-74-0.gif)
916211-74-0
13 suppliers
$445.00/250mg
Yield:916211-74-0 72% ,916211-75-1 6%
Reaction Conditions:
in methanol; for 5 h;Heating / reflux;
Steps:
13
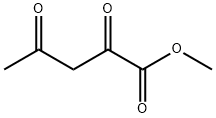
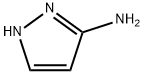
Preparative Example 13. To a solution of commercially available lH-pyrazol-5 -amine (86.4 g) in MeOH (1.80 L) was added commercially available methyl acetopyruvate (50.0 g). The mixture was heated to reflux for 5 h and then cooled to room temperature overnight. The precipitated yellow needles were collected by filtration and the supernatant was concentrated at 4O0C under reduced pressure to ~2/i volume until more precipitate began to form. The mixture was cooled to room temperature and the precipitate was collected by filtration. This concentration/ precipitation/filtration procedure was repeated to give 3 batches. This material was combined and recrystallized from MeOH to give the major isomer, methyl 7- methyl-pyrazolo[l,5-a]pyrimidine-5-carboxylate (81.7 g, 72%). [MH]+ = 192.The remaining supernatants were combined, concentrated and purified by chromatography (silica, cyclohexane/EtOAc) to afford the minor isomer, methyl 5-methyl-pyrazolo[l,5-a]pyrimidine-7-carboxylate (6.8 g, 6%). [MH]+ = 192.
References:
WO2008/63671,2008,A2 Location in patent:Page/Page column 131
1820-80-0
477 suppliers
$5.00/5g

20577-61-1
180 suppliers
$14.00/1g
![Methyl 7-Methylpyrazolo[1,5-a]pyriMidine-5-carboxylate](/CAS/20150408/GIF/916211-74-0.gif)
916211-74-0
13 suppliers
$445.00/250mg