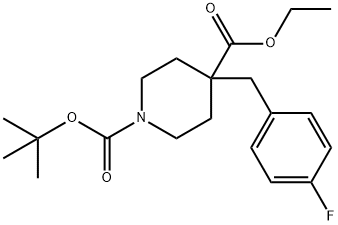
ETHYL N-BOC-4-(4-FLUOROBENZYL)PIPERIDINE-4-CARBOXYLATE synthesis
- Product Name:ETHYL N-BOC-4-(4-FLUOROBENZYL)PIPERIDINE-4-CARBOXYLATE
- CAS Number:917755-77-2
- Molecular formula:C20H28FNO4
- Molecular Weight:365.44
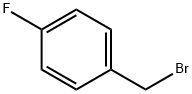
459-46-1
327 suppliers
$10.00/1g
142851-03-4
297 suppliers
$5.00/1g

917755-77-2
23 suppliers
$234.00/1g
Yield:917755-77-2 89.2%
Reaction Conditions:
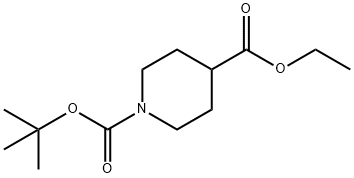
Stage #1: 1-tert-butyl 4-ethyl piperidine-1,4-dicarboxylatewith lithium diisopropyl amide in tetrahydrofuran at -78 - -40; for 1 h;
Stage #2: 4-Fluorobenzyl bromide in tetrahydrofuran at -78; for 2 h;Product distribution / selectivity;
Steps:
82.1
Step-1: 4-(4-Fluoro-benzyl)-piperidine-1,4-dicarboxylic acid 1-tent-butyl ester 4-ethyl ester; To a stirred solution of lithium diisopropyl amide in THF was added Piperidine-1,4-dicarboxylic acid 1-tert-butyl ester 4-ethyl ester (2 g, 7.7 mmol) at -78° C. The reaction mixture was stirred at -40° C. for 1 hr. Then reaction mixture was cooled to -78° C. and was added 4-fluorobenzyl bromide (1.46 g, 7.7 mmole) was added to reaction mass at -78° C. The resulting mixture was stirred at -78° C. for 2 hrs. Then reaction mixture was quenched with saturated ammonium chloride solution and extracted with ethyl acetate. Organic layer was washed with water and brine, dried over Na2SO4 and concentrated under reduced pressure. The crude mass was washed with hexane to afford 2.5 g (89.2%) of 4-(4-Fluoro-benzyl)-piperidine-1,4-dicarboxylic acid 1-tert-butyl ester 4-ethyl ester. LC/MS [M+H]+: 366.3.
References:
US2010/152160,2010,A1 Location in patent:Page/Page column 77