
2-Chloro-4-((5-methyl-5H-pyrrolo[3,2-d]pyrimidin-4-yl)oxy)aniline synthesis
- Product Name:2-Chloro-4-((5-methyl-5H-pyrrolo[3,2-d]pyrimidin-4-yl)oxy)aniline
- CAS Number:919278-09-4
- Molecular formula:C13H11ClN4O
- Molecular Weight:274.71
![4-Chloro-5-Methyl-5H-pyrrolo[3,2-d]pyriMidine](/CAS/GIF/871024-38-3.gif)
871024-38-3
45 suppliers
$65.00/100mg
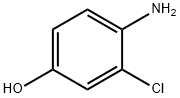
17609-80-2
316 suppliers
$39.00/5g
![2-Chloro-4-((5-methyl-5H-pyrrolo[3,2-d]pyrimidin-4-yl)oxy)aniline](/CAS/20180703/GIF/919278-09-4.gif)
919278-09-4
4 suppliers
inquiry
Yield:919278-09-4 36%
Reaction Conditions:
with potassium carbonate in 1-methyl-pyrrolidin-2-one at 120; for 18 h;
Steps:
3
A mixture of 4-chloro-5-methyl-5H-pyrrolo [3, 2- d] pyrimidine (168 mg, 1.0 mmol), 4-amino-3-chlorophenol (215 mg, 1.5 mmol), potassium carbonate (415 mg, 3.0 mmol) and N- methylpyrrolidone (3 mL) was stirred at 1200C for 18 hr . The reaction mixture was diluted with water and extracted with ethyl acetate (χ3) . The organic layer was concentrated under reduced pressure, and the residue was purified by column chromatography (NH silica gel, hexane/ethyl acetate=90/10_>0/100) to- give the title compound (100 mg, 36%).. 1H-NMR (CDCl3, 300 MHz) δ 4.05 (2H, br s) , 4.13 (3H, s) , 6.64 (IH, d, J = 3.0 Hz), 6.84 (IH, d, J = 8.7 Hz), 7.00 (IH, dd, J = 2.4, 8.7 Hz), 7.20 (IH, d, J = 2.4 Hz), 7.31 (IH, d, J = 3.0 Hz) , 8.45 (IH, s) .
References:
WO2007/4749,2007,A1 Location in patent:Page/Page column 112