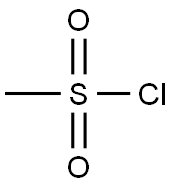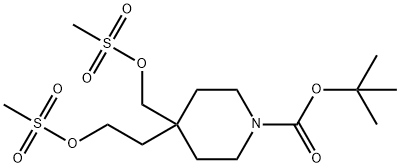
1-Piperidinecarboxylic acid, 4-[2-[(Methylsulfonyl)oxy]ethyl]-4-[[(Methylsulfonyl)oxy]Methyl]-, 1,1-diMethylethyl ester synthesis
- Product Name:1-Piperidinecarboxylic acid, 4-[2-[(Methylsulfonyl)oxy]ethyl]-4-[[(Methylsulfonyl)oxy]Methyl]-, 1,1-diMethylethyl ester
- CAS Number:929301-95-1
- Molecular formula:C15H29NO8S2
- Molecular Weight:415.52
Yield:929301-95-1 96%
Reaction Conditions:
with triethylamine in dichloromethane at 0; for 2 h;
Steps:
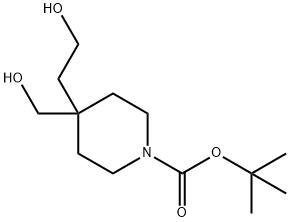
Preparation of compound 4To a solution of compound 3 (11O g, 0.43 mol) and Et3N (300 g, 412 mL, 3.0 mol) in CH2Cl2 (1100 mL) was added dropwise MsCl (170 g, 117 mL, 1.49 mol) in an ice bath. After the addition, the mixture was stirred at the same temperature for 2 h. TLC showed the reaction was complete. The mixture was poured into ice-water (200 mL) and stirred for 10 min. The organic layer was separated and the aqueous layer was extracted with CH2Cl2 two times. The combined organic layers were washed with 1 N aq. HCl (200 mLX 3) and brine, dried over MgSO4 and concentrated to give compound 4 (170 g, 96%) as brown syrup.
References:
WO2007/30061,2007,A1 Location in patent:Page/Page column 86; 87
3612-20-2
482 suppliers
$6.00/10g
![1-Piperidinecarboxylic acid, 4-[2-[(Methylsulfonyl)oxy]ethyl]-4-[[(Methylsulfonyl)oxy]Methyl]-, 1,1-diMethylethyl ester](/CAS/GIF/929301-95-1.gif)
929301-95-1
6 suppliers
inquiry
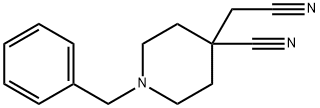
86945-27-9
26 suppliers
inquiry
![1-Piperidinecarboxylic acid, 4-[2-[(Methylsulfonyl)oxy]ethyl]-4-[[(Methylsulfonyl)oxy]Methyl]-, 1,1-diMethylethyl ester](/CAS/GIF/929301-95-1.gif)
929301-95-1
6 suppliers
inquiry

40117-92-8
16 suppliers
$439.00/1g
![1-Piperidinecarboxylic acid, 4-[2-[(Methylsulfonyl)oxy]ethyl]-4-[[(Methylsulfonyl)oxy]Methyl]-, 1,1-diMethylethyl ester](/CAS/GIF/929301-95-1.gif)
929301-95-1
6 suppliers
inquiry
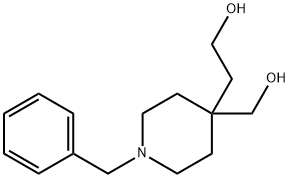
40118-06-7
1 suppliers
inquiry
![1-Piperidinecarboxylic acid, 4-[2-[(Methylsulfonyl)oxy]ethyl]-4-[[(Methylsulfonyl)oxy]Methyl]-, 1,1-diMethylethyl ester](/CAS/GIF/929301-95-1.gif)
929301-95-1
6 suppliers
inquiry