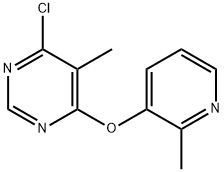
4-Chloro-5-methyl-6-(2-methylpyridin-3-yloxy)pyrimidine synthesis
- Product Name:4-Chloro-5-methyl-6-(2-methylpyridin-3-yloxy)pyrimidine
- CAS Number:930093-72-4
- Molecular formula:C11H10ClN3O
- Molecular Weight:235.67
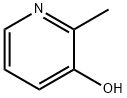
1121-25-1
366 suppliers
$8.00/250mg
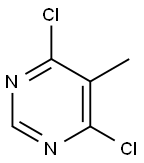
4316-97-6
199 suppliers
$8.00/1g

930093-72-4
30 suppliers
$205.00/250mg
Yield:930093-72-4 89.5%
Reaction Conditions:
Stage #1: 2-methyl-3-pyridinol;4,6-dichloro-5-methylpyrimidine in ISOPROPYLAMIDE at -8; for 1 h;
Stage #2: with potassium carbonate in ISOPROPYLAMIDE at -7.2 - 20; for 5 h;
Steps:
4.4.2.A
Step A: Preparation of 4-chloro-5-methyl-6-(2-methyl-pyridin-3-yloxy)-pyrimidine. [Show Image] To a mixture of 2-methyl-pyridin-3-ol (2g, 18.32mmol) and 4,6-dichloro-5-methylpyrimidine (2.98g, 18.32mmol) was added DMA (15mL). The resulting solution was stirred for one hour at -8°C, and potassium carbonate (2.53g, 18.32mmol) was introduced in one portion with no significant exothermic reaction was detected (temperature after addition was -7.2°C). The mixture was allowed to warm to room temperature (two hours), and then was stirred for an additional three hours (the progress of the reaction was monitored using LCMS). The crude mixture was cooled to 0°C; cooled water (3°C, 15mL) was slowly added. The temperature rose to 16°C, the solid was filtered at 1.7°C and washed three times with cooled water (3°C, 3x15mL) and dried in vacuum oven at 50°C for 24 hours. The solid was collected to give 4-chloro-5-methyl-6-(2-methyl-pyridin-3-yloxy)-pyrimidine (3.5833g, 89.5%). LCMS: 236-238 (MH)+, 200.1, 155-1557, 119.2. NMR (400MHz, δ ppm, DMSO d6): 8.4 (1H, s), 8 (1H, dd, J), 7.65 (1H, dd, J), 7.35 (1H, dd, J), 2.42 (3H, s), 2.3 (3H, s).
References:
EP1931654,2009,B1 Location in patent:Page/Page column 17