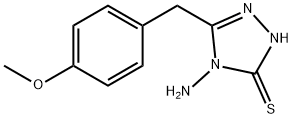
4-AMINO-5-(4-METHOXYBENZYL)-4H-1,2,4-TRIAZOLE-3-THIOL synthesis
- Product Name:4-AMINO-5-(4-METHOXYBENZYL)-4H-1,2,4-TRIAZOLE-3-THIOL
- CAS Number:93073-14-4
- Molecular formula:C10H12N4OS
- Molecular Weight:236.29
Yield:93073-14-4 75%
Reaction Conditions:
Stage #1: carbon disulfide;2-(4-methoxyphenyl)acetic acid hydrazidewith potassium hydroxide in methanol;Reflux;
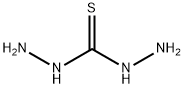
Stage #2: with hydrazine hydrate in water;Reflux;
Steps:
3; 4 Synthesis of 4-amino-3- (4-methoxybenzyl) -1H-1,2,4-triazole-5 (4H) -thione (Compound 1e)
0.125 mol of 2- (4-methoxyphenyl) acetohydrazide (compound 1c) was added to a solution of 0.125 mol of potassium hydroxide (KOH) dissolved in 50 mL of dry methanol, followed by cooling on ice. To this was added 0.125 mol of carbon disulfide for 2 to 3 hours Was added little by little while stirring continuously. As a result, Potassium-2- [2- (4-methoxyphenyl) acetyl] hydrazine carbodithioate (Compound 1d) was filtered off, washed with cold diethyl ether and dried. It was used immediately in the next step without purification.2- (2- (4-methoxyphenyl) acetyl] hydrazinecarbodithioate (compound 1d) was added to 20 mL of deionized water and 0.250 mol of hydrazine hydrate was added thereto, followed by refluxing for 8-10 hours. The reaction mixture turned yellowish green with the generation of hydrozenesulfide and finally homogenized. The mixture was then poured into crushed ice and acidified by addition of hydrochloric acid (HCl). The resulting 4-amino-3- (4-methoxybenzyl) -1-hydrogen- -thiazole-5 (4H) -thione (Compound 1e) After cooling, the mixture was washed with cold water and crystallized with aqueous methanol to obtain a white solid (Compound 1e) (75%).
References:
KR2017/4492,2017,A Location in patent:Paragraph 018; 0123
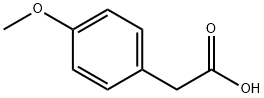
104-01-8
514 suppliers
$6.00/10g

93073-14-4
10 suppliers
inquiry
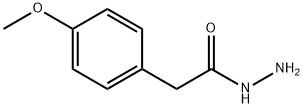
57676-49-0
57 suppliers
inquiry

93073-14-4
10 suppliers
inquiry
4693-91-8
90 suppliers
$19.00/1g

93073-14-4
10 suppliers
inquiry