
N*1*-(4-Methoxy-benzyl)-N*1*-Methyl-ethane-1,2-diaMine synthesis
- Product Name:N*1*-(4-Methoxy-benzyl)-N*1*-Methyl-ethane-1,2-diaMine
- CAS Number:933737-03-2
- Molecular formula:C11H18N2O
- Molecular Weight:194.27
![Acetonitrile, 2-[[(4-methoxyphenyl)methyl]methylamino]-](/CAS/20180703/GIF/127780-69-2.gif)
127780-69-2
0 suppliers
inquiry

933737-03-2
7 suppliers
$635.00/1g
Yield:933737-03-2 80%
Reaction Conditions:
Stage #1: 2-((4-methoxybenzyl)(methyl)amino)acetonitrilewith lithium aluminium tetrahydride in tetrahydrofuran at -20 - 20; for 2 h;
Stage #2: with water;Rochelle's salt at -10;
Steps:
24.3 Step 3. N1-4-methoxybenzyl-N1-methylethanediamine
Step 3. N1-4-methoxybenzyl-N1-methylethanediamine PMB^ - NH2 / [00315] 1M LiAlH4 in THF (1.10 L, 1.10 mol) was added to THF (1 L) and the solution cooled to -20 °C. A solution of [(4-methoxybenzyl)methylamino]acetonitrile (140 g, 0.737 mol) in THF (500 mL) was added over 1 h while maintaining the reaction temperature at < -10 °C. The mixture was allowed to warm to room temperature and stirred 1 h. The mixture was cooled to -10 °C and a solution of sodium potassium tartrate (37% w/v, 200 mL) was added dropwise cautiously while maintaining reaction temperature at < 0 °C. Celite (50 g) was added and the mixture filtered through a pad of Celite, washing the solids with THF (300 mL) followed by MTBE (300 mL). The filtrate was dried over Na2S04, filtered and the solution concentrated under reduced pressure. The residual liquid was Kugelrohr distilled to give 114 g (80%) of N1-4-methoxybenzyl-N1-methylethanediamine (oven temperature 128- 135°, 1.0 Torr).
References:
WO2014/66834,2014,A1 Location in patent:Page/Page column 00315
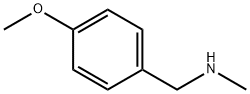
702-24-9
181 suppliers
$12.00/1g

933737-03-2
7 suppliers
$635.00/1g
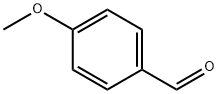
123-11-5
884 suppliers
$10.00/10g

933737-03-2
7 suppliers
$635.00/1g