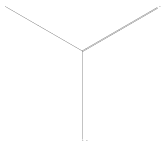
1-Pyrrolidinecarboxylic acid, 3-(acetylthio)-, 1,1-dimethylethyl ester, (3R)- synthesis
- Product Name:1-Pyrrolidinecarboxylic acid, 3-(acetylthio)-, 1,1-dimethylethyl ester, (3R)-
- CAS Number:935845-19-5
- Molecular formula:C11H19NO3S
- Molecular Weight:245.34
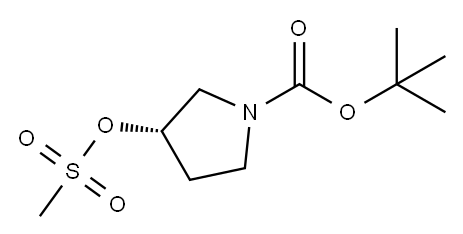
132945-75-6
47 suppliers
$9.00/250mg

10387-40-3
358 suppliers
$10.00/1g

935845-19-5
4 suppliers
inquiry
Yield:935845-19-5 92%
Reaction Conditions:
in N,N-dimethyl-formamide at 65;
Steps:
2.2.d
[00243] To a cooled (-5 to-10°C, ice/salt bath) solution of pyrrolidinol (xiv) (18.2 g, 98 mmol) and triethylamine (28 ml, 196 mmol) in ethylacetate (150 ml) was slowly added methanesulfonyl chloride (9.1 ml, 118 mmol) via a syringe. After completion of addition, the mixture was left stirring at room temperature for one hour. Water (100 ml) was added, and the layers were separated. The organic layer was washed with 1 N aq. HCl solution (100 ml), 5% aq. sodium bicarbonate solution (100 ml), and with saturated aqueous NaCl solution (100 ml). Then, the organic layer was dried over sodium sulfate, filtered, and evaporated to dryness under reduced pressure, yielding 24.3 g (92 mmol, 94%) of a yellow oil. This oil was dissolved in 150
References:
WO2007/50522,2007,A1 Location in patent:Page/Page column 57-58

127423-61-4
66 suppliers
$15.00/100mg

10387-40-3
358 suppliers
$10.00/1g

935845-19-5
4 suppliers
inquiry
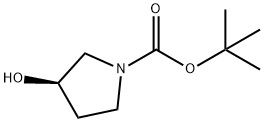
109431-87-0
364 suppliers
$5.00/1g

935845-19-5
4 suppliers
inquiry
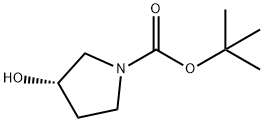
101469-92-5
356 suppliers
$6.00/5g

935845-19-5
4 suppliers
inquiry