
Methyl 2,6-dioxo-5-(trifluoromethyl)-1,2,3,6-tetrahydro-4-pyrimidinecarboxylate synthesis
- Product Name:Methyl 2,6-dioxo-5-(trifluoromethyl)-1,2,3,6-tetrahydro-4-pyrimidinecarboxylate
- CAS Number:936476-63-0
- Molecular formula:C7H5F3N2O4
- Molecular Weight:238.12
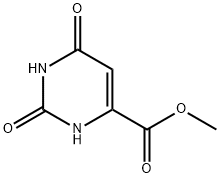
6153-44-2
123 suppliers
$7.00/250mg
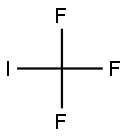
2314-97-8
183 suppliers
$40.00/25g

936476-63-0
13 suppliers
$325.00/500mg
Yield:936476-63-0 80%
Reaction Conditions:
with ferrocene;sulfuric acid;dihydrogen peroxide in water;dimethyl sulfoxide at 60 - 70; for 0.333333 h;
Steps:
15 EXAMPLE 15
0.17 g (1.0 mmol) of 6-methoxycarbonyluracil and 0.058 g (0.3 mmol) of ferrocene were weighed and placed in a 50 ml two-neck flask equipped with a magnetic rotor and the atmosphere in the flask was replaced with argon. The following materials were added thereinto: 1.8 ml of dimethyl sulfoxide, 2.0 ml of a 1N dimethyl sulfoxide solution of sulfuric acid, 1.0 ml of a 3.0 mol/l dimethyl sulfoxide solution of trifluoromethyl iodide and 0.2 ml of a 30% hydrogen peroxide aqueous solution. The mixture was stirred at 60 to 70°C for 20 minutes and then the resulting solution was cooled to room temperature. Formation of 6-methoxycarbonyl-5-trifluoromethyluracil (19F-NMR yield: 84%) was confirmed by 19F-NMR with 2,2,2-trifluoroethanol as an internal standard. 6-Methoxycarbonyl-5-trifluoromethyluracil was obtained as a white solid (0.20 g, yield: 80%) by column chromatography. 1H-NMR (deuterated acetone): δ3.94(s, 3H), 10.70(s, 1H), 11.10 (brs, 1H). 13C-NMR (deuterated acetone): δ54.5, 100.8 (q, JCF=32.2Hz), 123.1 (q, JCF=269.7Hz), 147.4 (q, JCF=3.52Hz), 149.9, 160. 1, 161.6. 19F-NMR (deuterated acetone): δ-60.6. MS (m/z): 238 [M]+.
References:
EP1947092,2008,A1 Location in patent:Page/Page column 39