
6-CHLORO-2-OXO-4-PHENYL-1,2-DIHYDRO-QUINOLINE-3-CARBOXYLIC ACID ETHYL ESTER synthesis
- Product Name:6-CHLORO-2-OXO-4-PHENYL-1,2-DIHYDRO-QUINOLINE-3-CARBOXYLIC ACID ETHYL ESTER
- CAS Number:93654-27-4
- Molecular formula:C18H14ClNO3
- Molecular Weight:327.76
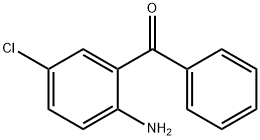
719-59-5
522 suppliers
$10.00/5g
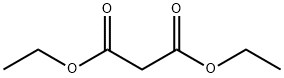
105-53-3
735 suppliers
$5.00/25g

93654-27-4
17 suppliers
$90.00/250mg
Yield:93654-27-4 95%
Reaction Conditions:
with sodium triflate;acetic acid in neat (no solvent) at 110; for 3 h;Friedlaender Quinoline Synthesis;
Steps:
5.3 General procedure for the synthesis of substituted ethyl 2-oxo-4-phenyl-1,2-dihydroquinoline-3-carboxylate (3f, 3g)
To a round bottom flask substituted 2-aminobenzophenone (0.01mol), 10mol % sodium trifluoromethanesulfonate, 5mol % acetic acid, and diethyl malonate (0.012mol) added, and refluxed at 110°C till the reaction get completed. After completion of the reaction, the reaction mixture was adsorbed and loaded on silica gel (100-200) mesh column. The column was eluted with 15-25% ethyl acetate in hexane to give desired compound in yield ranging from 91 to 95%.
References:
Kumar, Gautam;Sathe, Asawari;Krishna, Vagolu Siva;Sriram, Dharmarajan;Jachak, Sanjay M. [European Journal of Medicinal Chemistry,2018,vol. 157,p. 1 - 13]

110-89-4
3 suppliers
$15.96/100ML
108-20-3
543 suppliers
$25.00/25mL

719-59-5
522 suppliers
$10.00/5g

105-53-3
735 suppliers
$5.00/25g

93654-27-4
17 suppliers
$90.00/250mg
![Propanoic acid,3-[(4-chlorophenyl)amino]-3-oxo-, ethyl ester](/CAS/GIF/15386-84-2.gif)
15386-84-2
8 suppliers
inquiry

93654-27-4
17 suppliers
$90.00/250mg

106-47-8
465 suppliers
$5.00/5G

93654-27-4
17 suppliers
$90.00/250mg
36239-09-5
277 suppliers
$12.00/5g

93654-27-4
17 suppliers
$90.00/250mg