
1-(6-Chloro-2-methylpyrimidin-4-yl)-4-hydroxypiperidine synthesis
- Product Name:1-(6-Chloro-2-methylpyrimidin-4-yl)-4-hydroxypiperidine
- CAS Number:936845-82-8
- Molecular formula:C10H14ClN3O
- Molecular Weight:227.69
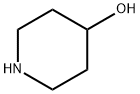
5382-16-1
13 suppliers
$9.00/5G
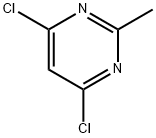
1780-26-3
473 suppliers
$10.00/10g

936845-82-8
12 suppliers
$60.00/50mg
Yield:936845-82-8 98%
Reaction Conditions:
Stage #1: 4-HYDROXYPIPERIDINE;2,4-dichloro-2-methylpyrimidinewith N-ethyl-N,N-diisopropylamine in 1,4-dioxane at 20; for 72 h;
Stage #2: with sodium hydrogencarbonate in water;ethyl acetate;
Steps:
21
To a solution of 4,6-dichloro-2-methylpyrimidine (2.73 g, 16.7 mmol) in 1,4- dioxane (200 mL) was added piperidin-4-ol (1.13 g, 11.1 mmol) and NN- diisopropylethylamine (7.9 mL, 45 mmol). The reaction mixture was stirred 72 h at room temperature. The solvent was removed in vacuo. The residue was dissolved in EtOAc (150 mL) and washed with saturated NaHCO3 (2 x 50 mL). The organic layer was dried (Na2SO4). The solvent was removed in vacuo to afford the crude product (2.5 g, 98%) as yellow oil.
References:
WO2007/56023,2007,A2 Location in patent:Page/Page column 42-43