4-AMinopyrrolo[1,2-f][1,2,4]triazine-6-carbonitrile synthesis
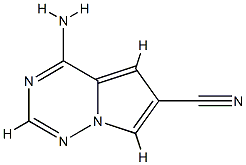
- Product Name:4-AMinopyrrolo[1,2-f][1,2,4]triazine-6-carbonitrile
- CAS Number:937049-27-9
- Molecular formula:C7H5N5
- Molecular Weight:159.1481
Yield:937049-27-9 75%
Reaction Conditions:
with potassium carbonate in ethanol at 80; for 2 h;
Steps:
AX.3
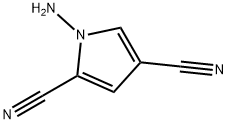
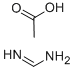
To a solution of EtOH (50 mL) was added 1 -amino- lH-pyrrole-2,4-dicarbonitrile (1.00 g, 7.57 mmol) followed by formamidine acetate (9.46 g, 90.8 mmol) and potassium carbonate (14.6 g, 106.0 mmol). The solution was heated to 80 0C for 2 h. Upon cooling to it, the reaction mixture was diluted with EtOAc transferred to a separatory funnel and washed with water. The organic layer was collected, dried (MgSO4), filtered, and concentrated to dryness. The crude material was then triturated with EtOH- water. The solid was collected, washed with water, and dried under vacuum to afford 0.91 g of the above compound as a yellow-orange solid (5.70 mmol, yield 75%). 1H-NMR (DMSO-^6) δ 8.37 (d, / = 1.8 Hz, IH), 8.19 (d, / = 17.4, 2H), 7.93 (s, IH), 7.27 (d, J = 1.8 Hz, IH); LCMS RT = 1.14 min.
References:
WO2007/64883,2007,A2 Location in patent:Page/Page column 236