
ethyl 1-(4-methoxybenzyl)-4-oxo-1,4-dihydroquinoline-3-carboxylate synthesis
- Product Name:ethyl 1-(4-methoxybenzyl)-4-oxo-1,4-dihydroquinoline-3-carboxylate
- CAS Number:937268-26-3
- Molecular formula:C20H19NO4
- Molecular Weight:337.37
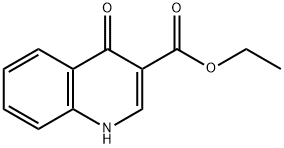
52980-28-6
128 suppliers
$12.00/250mg
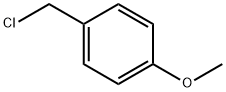
824-94-2
448 suppliers
$24.19/5 g

937268-26-3
10 suppliers
$380.00/1g
Yield: 62%
Reaction Conditions:
with potassium carbonate in N,N-dimethyl-formamide at 40 - 80; for 18 h;
Steps:
39 Compound 151Ethyl 1 -(4-methoxybenzyl)-4-oxo- 1 ,4-dihydroquinoline-3 -carboxylate
To a suspension of ethyl-4-oxo-1,4-dihydroquinoline-3-carboxylate (lg, 4.60 mmol) and K2C03 (1.20 g, 8.60 mmol) in DMF (7 mL) at 40 °C was added 4-methoxybenzyl chloride (2.28 mL, 16.75 mmol) dropwise. The reaction mixture was stirred at 80 °C for 18 h. Solvent was removed under reduced pressure and the product purified by flash chromatography using a gradient of 0-20% MeOH in EtOAc to give the titleproduct (960 mg, 62%) as a white powder.HRIVIS m/z (El) 337.13145, calculated for C2oH,9NO4 337.13086; ‘H NIVIR (500IVIHz, CDC13) 8.68 (s, 1H, aromatic), 8.51 (dd, J = 8.2, 1.6, 1H, aromatic), 7.56(ddd, J= 8.7, 7.2, 1.6, 1H, aromatic), 7.43 - 7.37 (m, 2H, aromatic), 7.28 (d, J= 8.5,1H, aromatic), 7.10 (d, J = 8.7, 1H, aromatic), 6.85 (dd, J = 8.7, 3.5, 2H, aromatic),5.37 (s, 2H, Bn-CH2), 4.39 (q, J= 7.1, 2H, CH2CH3), 3.75 (s, 3H, O-CH3), 1.40 (t, J7.1, 3H, CH2CH3); ‘3C NIVIR (126 IVIFIz, CDC13) 174.54 (CO), 166.20 (CO), 159.97(aromatic), 149.87 (aromatic), 139.41 (aromatic), 132.97 (aromatic), 130.46(aromatic), 129.11 (aromatic), 128.83 (aromatic), 128.04 (aromatic), 127.90(aromatic), 126.13 (aromatic), 125.55 (aromatic), 116.84 (aromatic), 114.93(aromatic), 114.12 (aromatic), 61.34 (Bn-CH2), 57.31 (CH2CH3), 55.52 (O-CH3),14.62 (CH2CH3).
References:
UCL BUSINESS PLC;SELWOOD, David;HOBBS, Adrian WO2015/189560, 2015, A1 Location in patent:Page/Page column 109
2746-25-0
171 suppliers
$27.57/250mgs:

52980-28-6
128 suppliers
$12.00/250mg

937268-26-3
10 suppliers
$380.00/1g
87-13-8
443 suppliers
$5.00/5g

937268-26-3
10 suppliers
$380.00/1g

62-53-3
661 suppliers
$10.00/1g

937268-26-3
10 suppliers
$380.00/1g
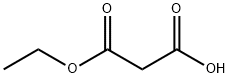
1071-46-1
295 suppliers
$5.00/1g

937268-26-3
10 suppliers
$380.00/1g