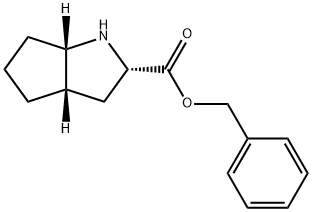
(S,S,S)-2-Azabicyclo[3,3,0]-octane-carboxylic acid benzylester hydrochloride synthesis
- Product Name:(S,S,S)-2-Azabicyclo[3,3,0]-octane-carboxylic acid benzylester hydrochloride
- CAS Number:93779-31-8
- Molecular formula:C15H19NO2
- Molecular Weight:245.32
![Cyclopenta[b]pyrrole-2-carboxylic acid, octahydro-, phenylmethyl ester, (2S,3aS,6aS)-, (αS)-α-hydroxybenzeneacetate (1:1)](/CAS/20211123/GIF/135214-79-8.gif)
135214-79-8
0 suppliers
inquiry
![(S,S,S)-2-Azabicyclo[3,3,0]-octane-carboxylic acid benzylester hydrochloride](/CAS/GIF/93779-31-8.gif)
93779-31-8
99 suppliers
inquiry
Yield:-
Reaction Conditions:
with sodium hydroxide in dichloromethane;water at 0 - 3; for 0.25 - 1 h;Product distribution / selectivity;
Steps:
3; 4
55 g of the S,S,S -diastereomeric salt of 2-azabicyclo[3,3,0]-octane-carboxylic acid benzyl ester of Formula III was taken into a round bottom flask containing 450 ml of dichloromethane. The mixture was stirred for 10 minutes at 25° C. A solution of 11.2 g of sodium hydroxide in 45 ml of water was added to the above reaction mass at 0-3° C. The reaction mixture was maintained at 0-3° C. for 15 minutes, and then the organic layer was separated. The resultant aqueous layer was washed with 165 ml of dichloromethane in three equal lots. The pH of the aqueous layer was adjusted to 2.5 using 15.4 ml of aqueous hydrochloric acid. The resultant reaction solution was stirred for about 3 hours at 0-3° C. The separated solid was filtered and washed with 110 ml of dichloromethane, and the wet solid was dried at 65° C. under a vacuum of 350 mm Hg for 7 hours to afford 36.2 g of title compound. Purity by HPLC: 100%; 64.5 kg of the compound obtained in Example 2 was added to a reactor containing 322 liters of dichloromethane. The mixture was stirred for 10 minutes and then cooled to 2° C. A solution of 13.12 kg of sodium hydroxide flakes in 53.8 liters of water was added to the above mixture at 2 to 3° C. and the reaction mass was stirred at the same temperature for one hour. The organic layer was separated and the aqueous layer was extracted into 3×64.5 liters of dichloromethane. The combined organic layer was taken into another reactor and 15.7 liters of 36% aqueous hydrochloric acid was added at 2 to 3° C. The reaction mass was maintained at 2 to 3° C. for 2 hours. The separated solid was filtered and washed with 64.5 liters of chilled dichloromethane. The wet solid was dried at 62° C. for 2 hours to yield 43 kg of the title compound. Purity by HPLC: 99.93%.
References:
Bolugoddu, Vijayabhaskar;Mamilla, Srinivas Reddy;Golla, Chinamala Kondaiah;Madduri, Srinivasa Reddy;Kodipyaka, Ravinder;Das, Surajit;Bhushan, Indu;Mohan, Mailatur Sivaraman US2007/232680, 2007, A1 Location in patent:Page/Page column 11