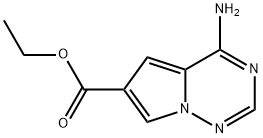
ETHYL 4-AMINOPYRROLO[2,1-F][1,2,4]TRIAZINE-6-CARBOXYLATE synthesis
- Product Name:ETHYL 4-AMINOPYRROLO[2,1-F][1,2,4]TRIAZINE-6-CARBOXYLATE
- CAS Number:939808-08-9
- Molecular formula:C9H10N4O2
- Molecular Weight:206.2
Yield:939808-08-9 49%
Reaction Conditions:
with sulfuric acid at 80;
Steps:
Ethyl 4-aminopyrrolo[2,1-f][1,2,4]triazine-6-carboxylate
A solution of 4-aminopyrrolo[2,l-fJ[l,2,4]triazine-6-carbonitrile (3.9 g, 24.5 mmol; preparation described in Int. Pat. Appl. WO 2007/064883) in ethanol (124.8 ml) was stirred with concentrated sulfuric acid (62.4 ml) at 80°C overnight. After cooling to rt, the reaction mixture was poured onto 800 g of ice and brought to pH 6-7 with concentrated aq. sodium hydroxide solution. Ethyl acetate (500 ml) and dichloromethane (500 ml) were added to the suspension, and the resulting mixture was filtered over kieselguhr. The organic layer was separated from the aqueous layer. The solid was dissolved in hot water (1 L), and the aqueous layer was extracted twice with ethyl acetate. The combined organic layers were dried over sodium sulfate and evaporated. The residue was triturated with an isopropanol/diethylether mixture, and the solid was filtered off yielding 2.5 g (49% of th.) of the title compound. LC-MS (method 2): Rt = 0.59 min; MS (ESIpos): m/z = 206 (M+H)+ 'H-NMR (400 MHz, DMSO-de): δ = 8.11-7.97 (m, 3H), 7.88 (s, 1H), 7.34 (br. s, 1H), 4.27 (q, 2H), 1.30 (t, 3H) ppm
References:
WO2013/87647,2013,A1 Location in patent:Page/Page column 49-50
![4-AMinopyrrolo[1,2-f][1,2,4]triazine-6-carbonitrile](/CAS/20180906/GIF/937049-27-9.gif)
