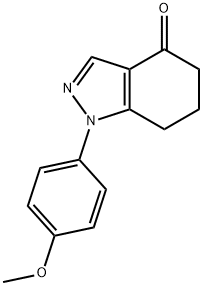
1-(4-METHOXYPHENYL)-1,5,6,7-TETRAHYDROINDAZOL-4-ONE synthesis
- Product Name:1-(4-METHOXYPHENYL)-1,5,6,7-TETRAHYDROINDAZOL-4-ONE
- CAS Number:945363-51-9
- Molecular formula:C14H14N2O2
- Molecular Weight:242.27

504-02-9
553 suppliers
$5.00/10g
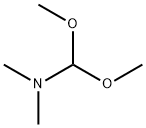
4637-24-5
590 suppliers
$5.00/25g

3471-32-7
31 suppliers
inquiry

945363-51-9
11 suppliers
inquiry
Yield:945363-51-9 90%
Reaction Conditions:
with 2,2,2-trifluoroethanol for 0.5 h;Reflux;regioselective reaction;
Steps:
4.2. General procedure for preparation of the arylpyrazole derivatives
Arylhydrazine (1.2 mmol) in TFE (0.5 ml) was slowly added to a solution of β-dicarbonyl (1.2 mmol) and N,N-dimethylformamide dimethyl acetal (1.2 mmol) in TFE (0.5 ml) at room temperature. The progress of reactions was monitored by TLC. After completion of the reaction solvent was evaporated under reduced pressure, and water (10 ml) was added to the residue and extracted with ethylacetate (2× 10 ml). The organic layer was successively washed with sodium hydrogen carbonate solution and water, and then dried with magnesium sulfate. Evaporation of the solvent afforded 4-carboxylate pyrazole. If necessary the products were further purified by column chromatography on silica gel.CommentReactions with 1,3-cyclohexanedione were carried out at reflux temperature (refPreviewPlaceHolderTable 2, entries 10-12). In the case of open-chain β-diketones, under optimal reaction conditions two equivalents of DMFDMA were used (refPreviewPlaceHolderTable 2, entries 13-18).
References:
Alinezhad, Heshmatollah;Tajbakhsh, Mahmood;Zare, Mahboobeh [Journal of Fluorine Chemistry,2011,vol. 132,# 11,p. 995 - 1000] Location in patent:experimental part
![2-[(Dimethylamino)methylene]-1,3-cyclohexanedione](/CAS/GIF/85302-07-4.gif)
85302-07-4
48 suppliers
$75.00/1g
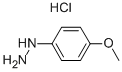
19501-58-7
377 suppliers
$7.00/250mg

945363-51-9
11 suppliers
inquiry

504-02-9
553 suppliers
$5.00/10g

945363-51-9
11 suppliers
inquiry