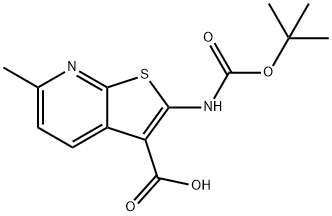
2-((TERT-BUTOXYCARBONYL)AMINO)-6-METHYLTHIENO[2,3-B]PYRIDINE-3-CARBOXYLIC ACID synthesis
- Product Name:2-((TERT-BUTOXYCARBONYL)AMINO)-6-METHYLTHIENO[2,3-B]PYRIDINE-3-CARBOXYLIC ACID
- CAS Number:953821-10-8
- Molecular formula:C14H16N2O4S
- Molecular Weight:308.35
Yield:953821-10-8 80%
Reaction Conditions:
Stage #1: tert-butyl (3-bromo-6-methylthieno[2,3-b]pyridin-2-yl)carbamatewith n-butyllithium in tetrahydrofuran;hexane at -78; for 1 h;
Stage #2: carbon dioxide in tetrahydrofuran;hexane at 0 - 10;
Stage #3: with hydrogenchloride in tetrahydrofuran;hexane;water;ethyl acetate;
Steps:
56
Reference Example 56 2-[(tert-butoxycarbonyl)amino]-6-methylthieno[2,3-b]pyridine-3-carboxylic acid To a solution of tert-butyl (3-bromo-6-methylthieno[2,3-b]pyridin-2-yl)carbamate obtained in Reference Example 55 (9.07 g, 26.4 mmol) in dry THF (88 mL) added dropwise 1.6 M n-butyllithium hexane solution (38 mL, 60.7 mmol) at -78°C under a nitrogen atmosphere, and the mixture was stirred at the same temperature for 1 hr. Dry carbon dioxide gas vaporized from solid carbon dioxide was bubbled at 0-10°C. The reaction mixture was diluted with water and ethyl acetate, 1N hydrochloric acid was added, and the precipitated solid was collected by filtration. The obtained solid was purified by repeatedly suspending and filtering using water and acetonitrile/diethyl ether (1:1) successively to give the title compound (6.51 g, yield 80%) as white crystals. melting point 162-163°C EI(pos) 309 [M+H]+ 1H NMR (DMSO-d6) δ1.53 (9H, s), 2.50 (3H, s), 7.29 (1H, d, J = 8.3 Hz), 8.37 (1H, d, J = 8.3 Hz), 11.26 (1H, br).
References:
EP2009011,2008,A1 Location in patent:Page/Page column 42; 43
![ethyl 3-amino-6-methylthieno[2,3-b]pyridine-2-carboxylate](/CAS/GIF/52505-51-8.gif)
52505-51-8
20 suppliers
$46.00/1g
![2-((TERT-BUTOXYCARBONYL)AMINO)-6-METHYLTHIENO[2,3-B]PYRIDINE-3-CARBOXYLIC ACID](/CAS/20180529/GIF/953821-10-8.gif)
953821-10-8
4 suppliers
inquiry
28900-10-9
237 suppliers
$9.00/1g
![2-((TERT-BUTOXYCARBONYL)AMINO)-6-METHYLTHIENO[2,3-B]PYRIDINE-3-CARBOXYLIC ACID](/CAS/20180529/GIF/953821-10-8.gif)
953821-10-8
4 suppliers
inquiry
![3-BROMO-6-METHYLTHIENO[2,3-B]PYRIDINE-2-CARBOXYLIC ACID](/CAS/20180601/GIF/953821-08-4.gif)
953821-08-4
5 suppliers
inquiry
![2-((TERT-BUTOXYCARBONYL)AMINO)-6-METHYLTHIENO[2,3-B]PYRIDINE-3-CARBOXYLIC ACID](/CAS/20180529/GIF/953821-10-8.gif)
953821-10-8
4 suppliers
inquiry
![Ethyl 3-Bromo-6-methylthieno[2,3-b]pyridine-2-carboxylate](/CAS/20210305/GIF/953821-07-3.gif)
953821-07-3
1 suppliers
inquiry
![2-((TERT-BUTOXYCARBONYL)AMINO)-6-METHYLTHIENO[2,3-B]PYRIDINE-3-CARBOXYLIC ACID](/CAS/20180529/GIF/953821-10-8.gif)
953821-10-8
4 suppliers
inquiry
![TERT-BUTYL (3-BROMO-6-METHYLTHIENO[2,3-B]PYRIDIN-2-YL)CARBAMATE](/CAS/20180527/GIF/953821-09-5.gif)
