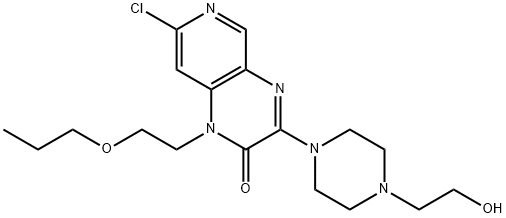
Pyrido[3,4-b]pyrazin-2(1H)-one, 7-chloro-3-[4-(2-hydroxyethyl)-1-piperazinyl]-1-(2-propoxyethyl)- synthesis
- Product Name:Pyrido[3,4-b]pyrazin-2(1H)-one, 7-chloro-3-[4-(2-hydroxyethyl)-1-piperazinyl]-1-(2-propoxyethyl)-
- CAS Number:954138-53-5
- Molecular formula:C18H26ClN5O3
- Molecular Weight:395.88
Yield:954138-53-5 83%
Reaction Conditions:
with triethylamine in tetrahydrofuran at 20; for 1 h;
Steps:
5.6
A solution of 3,7-dichloro-1-(2-propoxyethyl)pyrido[3,4-b]pyrazin-2(1H)-one (200 mg, 0.66 mmol), 1-(2-hydroxyethyl)piperazine (117 mg, 0.90 mmol, Aldrich) and triethylamine (0.27 mL, 1.94 mmol) in THF (3 mL) was stirred at room temperature for one hour. The reaction was partitioned between ethyl acetate and water. The layers were separated and the aqueous layer was extracted with ethyl acetate. The combined organic layers were washed with brine, dried over magnesium sulfate, and solvent was removed at reduced pressure to give 7-chloro-3-[4-(2-hydroxyethyl)piperazin-1-yl]-1-(2-propoxyethyl)pyrido[3,4-b]pyrazin-2(1H)-one. (218 mg, 83% yield). 1H NMR (CDCl3) δ 8.48 (1H), 7.34 (1H), 4.33-4.30 (2H), 4.04-4.00 (4H), 3.76-3.72 (2H), 3.69-3.65 (2H), 3.38-3.34 (2H), 2.67-2.64 (4H), 2.62-2.58 (2H), 1.55-1.48 (2H), 0.87-0.82 (3H).
References:
US2007/249615,2007,A1 Location in patent:Page/Page column 35
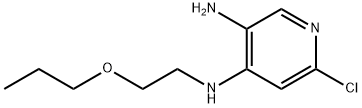
915307-80-1
3 suppliers
inquiry
![Pyrido[3,4-b]pyrazin-2(1H)-one, 7-chloro-3-[4-(2-hydroxyethyl)-1-piperazinyl]-1-(2-propoxyethyl)-](/CAS/20180702/GIF/954138-53-5.gif)
954138-53-5
6 suppliers
inquiry
![Pyrido[3,4-b]pyrazine-2,3-dione, 7-chloro-1,4-dihydro-1-(2-propoxyethyl)-](/CAS/20180703/GIF/1186508-07-5.gif)
1186508-07-5
3 suppliers
inquiry
![Pyrido[3,4-b]pyrazin-2(1H)-one, 7-chloro-3-[4-(2-hydroxyethyl)-1-piperazinyl]-1-(2-propoxyethyl)-](/CAS/20180702/GIF/954138-53-5.gif)
954138-53-5
6 suppliers
inquiry
5350-93-6
415 suppliers
$6.00/5g
![Pyrido[3,4-b]pyrazin-2(1H)-one, 7-chloro-3-[4-(2-hydroxyethyl)-1-piperazinyl]-1-(2-propoxyethyl)-](/CAS/20180702/GIF/954138-53-5.gif)
954138-53-5
6 suppliers
inquiry
![5-[N-(TERT-BUTOXYCARBONYL)AMINO]-2-CHLOROPYRIDINE](/CAS/GIF/171178-45-3.gif)
171178-45-3
128 suppliers
$8.00/250mg
![Pyrido[3,4-b]pyrazin-2(1H)-one, 7-chloro-3-[4-(2-hydroxyethyl)-1-piperazinyl]-1-(2-propoxyethyl)-](/CAS/20180702/GIF/954138-53-5.gif)
954138-53-5
6 suppliers
inquiry
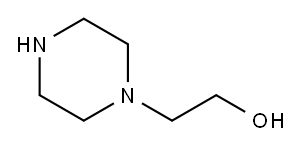
![3,7-Dichloro-1-(2-propoxyethyl)-1H,2H-pyrido-[3,4-b]pyrazin-2-one](/CAS/GIF/915307-81-2.gif)