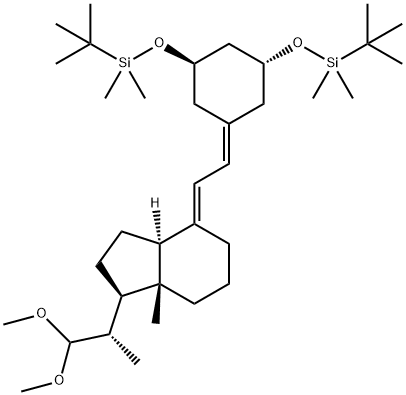
((1R,3R)-5-((E)-2-((1R,3aS,7aR)-1-((S)-1,1-dimethoxypropan-2-yl)-7a-methyldihydro-1H-inden-4(2H,5H,6H,7H,7aH)-ylidene)ethylidene)cyclohexane-1,3-diyl)bis(oxy)bis(tertbutyldimethylsilane) synthesis
- Product Name:((1R,3R)-5-((E)-2-((1R,3aS,7aR)-1-((S)-1,1-dimethoxypropan-2-yl)-7a-methyldihydro-1H-inden-4(2H,5H,6H,7H,7aH)-ylidene)ethylidene)cyclohexane-1,3-diyl)bis(oxy)bis(tertbutyldimethylsilane)
- CAS Number:957214-02-7
- Molecular formula:C35H66O4Si2
- Molecular Weight:607.07
![(1R,3aR,7aR)-1-[(1S)-2,2-DiMethoxy-1-Methylethyl]octahydro-7a-Methyl-4H-inden-4-one](/CAS/GIF/957214-01-6.gif)
957214-01-6
12 suppliers
$95.00/250mg

957214-02-7
10 suppliers
inquiry
Yield:957214-02-7 69%
Reaction Conditions:
Stage #1: (1R,3R)-1,3-bis((tert-butyldimethyl)silanyloxy)-5-[2-(diphenylphosphinoyl)-ethylidene]cyclohexanewith sodium hexamethyldisilazane in tetrahydrofuran at -78 - 0; for 0.0833333 h;
Stage #2: (1R,3aR,7aR)-1-((S)-1,1-dimethoxypropan-2-yl)-7a-methylhexahydro-1H-inden-4(2H)-one in tetrahydrofuran at -78 - 20; for 1.5 h;
Steps:
In a flame dried round bottom flask, cooled to -78 0C under argon, NaHMDS (2.574 mmol) was added to a solution of 16 (1.469 g, 2.574 mmol) in THF (30 ml_). The reaction vessel was suspended above the ice bath for 5 min, then recooled to -78 0C. A solution of 18 (0.6235 g, 2.451 mmol) in THF (10 ml.) was cannulated into the reaction mixture over a period of 15 min. The reaction mixture was left to stir at -78 0C for 1 h, followed by
References:
WO2007/131364,2007,A1 Location in patent:Page/Page column 24; 47-48
![(1R,3aR,7aR)-1-[(1S)-2,2-DiMethoxy-1-Methylethyl]octahydro-7a-Methyl-4H-inden-4-one](/CAS/GIF/957214-01-6.gif)
957214-01-6
12 suppliers
$95.00/250mg
![(3R-trans)-[2-[3,5-Bis[[(1,1-diMethylethyl)diMethylsilyl]oxy]cyclohexylidene]ethyl]diphenyl-phosphine Oxide](/CAS/GIF/139356-39-1.gif)
139356-39-1
88 suppliers
inquiry

957214-02-7
10 suppliers
inquiry
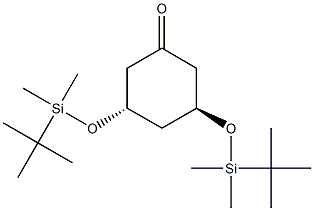
139356-35-7
6 suppliers
inquiry

957214-02-7
10 suppliers
inquiry
![(3R-trans)-[3,5-Bis[[(1,1-diMethylethyl)diMethylsilyl]oxy]cyclohexylidene]-acetic Acid Ethyl Ester](/CAS/GIF/139356-36-8.gif)
139356-36-8
12 suppliers
$139.90/2mg

957214-02-7
10 suppliers
inquiry
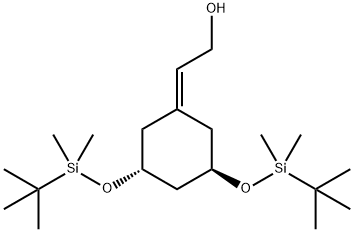
139356-37-9
44 suppliers
inquiry

957214-02-7
10 suppliers
inquiry