
2-Chloro-1-[2-(1-methylethyl)pyrazolo[1,5-a]pyridin-3-yl]-1-propanone synthesis
- Product Name:2-Chloro-1-[2-(1-methylethyl)pyrazolo[1,5-a]pyridin-3-yl]-1-propanone
- CAS Number:960355-08-2
- Molecular formula:C13H15ClN2O
- Molecular Weight:250.72
Yield:960355-08-2 84%
Reaction Conditions:
Stage #1: 2-isopropyl-pyrazolo[1,5-a]pyridinewith aluminum (III) chloride in dichloromethane at 15 - 20; for 0.416667 h;Inert atmosphere;
Stage #2: 2-chloropropionyl chloride in dichloromethane at 15 - 20; for 20.4167 h;Reflux;
Steps:
1.2.2 2-chloro-desmethylibudilast from IPPP
Step 2.2-Chloro-desmethylibudilast from IPPP A 5 L three-neck round bottom flask, equipped with a mechanical stirrer, thermocouple, reflux condenser and nitrogen inlet, was flushed with nitrogen and charged with dichloromethane (825 mL). Aluminum chloride (418 g, anhydrous) was charged to the reactor, with the aid of a dichloromethane rinse (150 mL). The mixture was stirred and cooled to 15° C. with an ice-water bath, and IPPP (2-isopropyl-pyrazolo[1,5-a]pyridine, 125 g, 0.78 mol) was added dropwise via an addition funnel over about a 15 minute period, with the temperature maintained at 15-20° C. Dichloromethane (100 mL) was used to rinse the residual IPPP from the funnel into the reactor. The mixture was stirred for 10 min at this temperature, then 2-chloropropionyl chloride (155 mL, 1.56 mol) was added dropwise via addition funnel over about a 10 minute period, with the temperature maintained at 15-20° C. Dichloromethane (100 mL) was used to rinse the residual 2-chloropropionyl chloride into the reactor. The cooling bath was removed and stirring was continued for 15 min, then the mixture was heated to reflux.After 20 h at reflux, the mixture was cooled to <20° C. and added slowly over about a 1 h period with stirring, to ice-water (1250 mL) cooled in an ice-water bath with the temperature maintained below 35° C. The resulting mixture was stirred at 15-20° C. for 10 min, then the dark brown organic layer was collected. The aqueous layer was washed with dichloromethane (2×125 mL). The combined organic extracts were stirred with 1 M NaOH (800 mL) for 30 min. The organic layer was separated and the aqueous layer was washed with dichloromethane (2×100 mL). The combined organic extracts were washed successively with water (500 mL, USP), saturated aqueous NaCl (650 mL) and then dried with stirring over anhydrous sodium sulfate. Silica gel (98 g) and activated carbon (DARCO, 29 g) were added and stirring was continued for 30 min. The dichloromethane was filtered through Celite and the drying agent and celite were rinsed with dichloromethane (3×400 mL). The mixture was concentrated by distillation at ambient pressure. When about 1.5 L of distillate was removed, heptane (1.6 L) was added and distillation was continued until the pot temperature reached 83° C. The mixture was allowed to cool slowly with stirring. After seeding and cooling to <10° C. for 1 h, the resulting precipitate were collected by filtration, rinsed with heptane (400 mL) and dried to obtain 165 g of chloroketone (84% yield) as an off-white solid.1H-NMR (DMSO-d6) δ 1.3 (m, 6H), 1.7 (d, 3H), 3.7 (m, 1H), 5.4 (q, 1H), 7.2 (t, 1H), 7.7 (t, 1H), 8.1 (d, 1H), 8.9 (d, 1H). HPLC: RT=11.5 min (100 area %).
References:
US2010/324082,2010,A1 Location in patent:Page/Page column 10-11
![2-Isopropylpyrazolo[1,5-a]pyridine-3-carboxylic acid](/CAS/20150408/GIF/126959-38-4.gif)
126959-38-4
11 suppliers
inquiry
![2-Chloro-1-[2-(1-methylethyl)pyrazolo[1,5-a]pyridin-3-yl]-1-propanone](/CAS/20210111/GIF/960355-08-2.gif)
960355-08-2
2 suppliers
inquiry
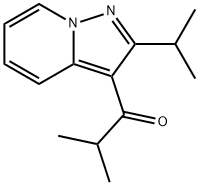
50847-11-5
319 suppliers
$8.00/10mg
![2-Chloro-1-[2-(1-methylethyl)pyrazolo[1,5-a]pyridin-3-yl]-1-propanone](/CAS/20210111/GIF/960355-08-2.gif)
960355-08-2
2 suppliers
inquiry

![Pyrazolo[1,5-a]pyridine, 2-(1-methylethyl)- (9CI)](/CAS/GIF/59942-84-6.gif)