
tert-butyl 3-(2-methoxy-4-nitrophenoxy)azetidine-1-carboxylate synthesis
- Product Name:tert-butyl 3-(2-methoxy-4-nitrophenoxy)azetidine-1-carboxylate
- CAS Number:960401-34-7
- Molecular formula:C15H20N2O6
- Molecular Weight:324.33
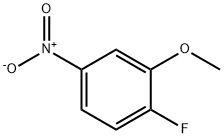
454-16-0
164 suppliers
$10.00/1g
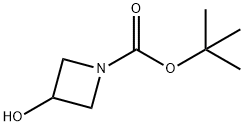
141699-55-0
385 suppliers
$6.00/1g

960401-34-7
11 suppliers
inquiry
Yield:960401-34-7 95%
Reaction Conditions:
with potassium-t-butoxide in tetrahydrofuran at 0; for 0.5 h;
Steps:
9
Dissolve l-fluoro-2-methoxy-4-nitro-benzene (118 g, 689 mmol) and 3-hydroxy- azetidine-1-carboxylic acid tert-bυXyl ester (125 g, 724 mmol) in THF (800 mL) and cool to 0 0C under nitrogen. To the above solution, add dropwise a 1 M THF solution of tert- BuOK (1 L). After the addition is complete, stir the dark brown solution for 30 min at 0 0C and then dilute with water (1 L) over a 10 min period. Stir the mixture for 5 min, then extract with tert-bvAyl methyl ether (2x ). Combine the organic solutions and wash with brine (2 x 700 mL), then dry and concentrate. Dry the solid in vacuo at 45 0C for 20 h to obtain 216 g (95%) of the title compound as a yellow solid. 1H NMR (300 MHz, CDCl3) & 6.47 (d, IH, J= 8.5), 6.30 (d, IH, J= 2.6), 6.17 (dd, IH, J= 2.4, 8.5), 4.76 (m, IH), 4.18 (m, 2H), 4.04 (m, 2H), 3.80 (s, 3H), 1.42 (s, 9H).
References:
WO2008/76562,2008,A1 Location in patent:Page/Page column 25