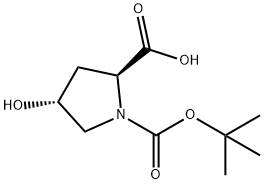
(2S,4R)-1-(tert-butoxycarbonyl)-4-(tosyloxy)pyrrolidine-2-carboxylic acid synthesis
- Product Name:(2S,4R)-1-(tert-butoxycarbonyl)-4-(tosyloxy)pyrrolidine-2-carboxylic acid
- CAS Number:96314-28-2
- Molecular formula:C17H23NO7S
- Molecular Weight:385.43
88043-21-4
107 suppliers
$17.00/1g

96314-28-2
13 suppliers
$59.00/2mg
Yield:96314-28-2 5175 g
Reaction Conditions:
with lithium hydroxide in water at 0 - 20;
Steps:
(2S,4R)-1-(tert-Butoxycarbonyl)-4-(tosyloxy)pyrrolidine-2-carboxylic acid
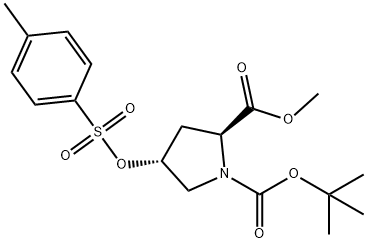
To a solution of (2S,4R)-1-tert-butyl 2-methyl 4-(tosyloxy)pyrrolidine-1,2-dicarboxylate (6950 g, 17.4 mol) in MeOH (70 L) was added a solution of LiOH (875 g, 20.8 mol) in H2O (35 L) at 0 °C. The mixture was stirred at room temperature overnight then poured into ice water (100 L) and extracted with EtOAc (2 × 30 L). The aqueous phase was acidified to pH 3 with 1M HCl, then extracted with EtOAc (2× 50 L). The combined organic layers were washed with brine, dried over Na2SO4 and concentrated in vacuo to give the title product (5175 g, 82% yield over two steps) as a white solid which was used for the next step without further purification. 1H NMR (CDCl3, 400 MHz): δ = 7.78 (d, J = 8.0 Hz, 2H), 7.36 (d, J = 7.6 Hz, 2H), 5.02 (s, 1H), 4.44- 4.37 (m, 1H), 3.76 - 3.51 (m, 2H), 2.46 - 2.22 (m, 5 H), 1.46 - 1.39 (m, 9H).
References:
Ahmed, Adil;Anderson, Niall A.;Benowitz, Andrew B.;Cryan, Jenni;Dai, Han;McGonagle, Grant A.;Rozier, Christine [Bioorganic and medicinal chemistry letters,2020] Location in patent:supporting information
51-35-4
838 suppliers
$6.00/5g

96314-28-2
13 suppliers
$59.00/2mg

98-59-9
588 suppliers
$9.00/5g

96314-28-2
13 suppliers
$59.00/2mg

24424-99-5
824 suppliers
$13.50/25G

96314-28-2
13 suppliers
$59.00/2mg