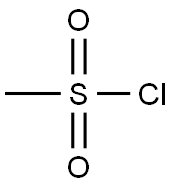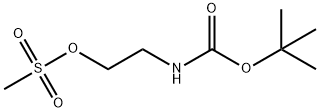
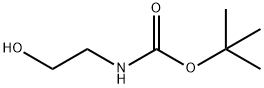
3-[(TERT-BUTOXYCARBONYL)AMINO]ETHYL METHANESULFONATE synthesis
- Product Name:3-[(TERT-BUTOXYCARBONYL)AMINO]ETHYL METHANESULFONATE
- CAS Number:96628-67-0
- Molecular formula:C8H17NO5S
- Molecular Weight:239.29
26690-80-2
324 suppliers
$6.00/5g
![3-[(TERT-BUTOXYCARBONYL)AMINO]ETHYL METHANESULFONATE](/CAS/GIF/96628-67-0.gif)
96628-67-0
16 suppliers
inquiry
Yield: 97%
Reaction Conditions:
with N-ethyl-N,N-diisopropylamine in dichloromethane
Steps:
8.a (Compound 30)
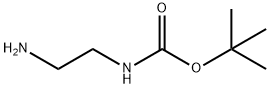
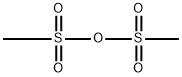
(a) A solution of tert-butyl 2-hydroxyethylcarbamate (25 mL, 161.6 mmol) in dichloromethane (60 mL) was cooled to 0° C. and stirred while first diisopropylethylamine (33.8 mL, 193.9 mmol) was added and then mesyl chloride (13.7 mL, 177.8 mmol) then was added dropwise. The mixture was allowed to warm to 23° C., stirred for 18 hours, poured into dichloromethane (200 mL) and washed with aqueous hydrochloric acid (3M, 3*25 mL) and saturated aqueous sodium hydrogencarbonate (2*25 mL). The organic layer was separated, dried (MgSO4) and concentrated in vacuo to provide tert-butyl 2-methylsulfonyloxyethylcarbamate (37.39 g, 97% yield) as a brown oil; MS (PB-PCI) C8H17NO5S m/e calc 239.08; found 240 (MH+).
References:
Church, Timothy J.;Cutshall, Neil Scott;Gangloff, Anthony R.;Jenkins, Thomas E.;Linsell, Martin S.;Litvak, Joane;Rice, Kenneth D.;Spencer, Jeffrey R.;Wang, Vivian R. US2001/53779, 2001, A1

24424-99-5
820 suppliers
$13.50/25G
![3-[(TERT-BUTOXYCARBONYL)AMINO]ETHYL METHANESULFONATE](/CAS/GIF/96628-67-0.gif)
96628-67-0
16 suppliers
inquiry