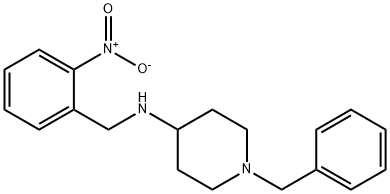
1-Benzyl-N-[(2-nitrophenyl)-methyl]piperidin-4-amine synthesis
- Product Name:1-Benzyl-N-[(2-nitrophenyl)-methyl]piperidin-4-amine
- CAS Number:98754-28-0
- Molecular formula:C19H23N3O2
- Molecular Weight:325.4
Yield:98754-28-0 12.8 kg
Reaction Conditions:
Stage #1: 4-amino-1-benzylpiperidine;2-nitro-benzaldehyde in methanol at 20; for 1.5 h;Inert atmosphere;Large scale;
Stage #2: with methanol;sodium tetrahydroborate at 0 - 20; for 2 h;Large scale;
Steps:
6.01.11.01 -Methyl-3-piperidin-4-yl-3,4-dihydro-lH-quinazolin-2-one 1 -Benzyl-piperidin-4-yl)-(2-nitro-benzyl)-amine
1 -Benzyl-piperidin-4-yl)-(2-nitro-benzyl)-amine 5955 g 2-nitrobenzaldehyde was suspended in 10 L methanol under nitrogen atmosphere and 7500 g 4-amino-l-benzyl-piperadine in 5 L methanol was added over 30 min. The reaction was stirred lh at RT. The reaction mixture was cooled to 0 °C and a cold solution of 1044 g sodium borohydride in 6425 mL water was added at a rate to keep the temperature below 10°C. After lh stirring at 0°C and lh at RT the reaction was coolded again to 0°C and aqueous 4 mol/L hydrochlorid acid was added. Then the reaction mixture was stirred at RT for 30 min and cooled again to 0-10 °C. Aqueous 5 mol/L sodium hydroxide solution was added until pH=14 and the reaction was extracted with tert-butyl methylether. The organic layer was washed with water and saturated sodium chloride solution and the solvend was evaporated. The residue was dissolved in toluol, filtered and concentrated again to yield 12.8 kg of the desired product.
References:
WO2013/83741,2013,A1 Location in patent:Page/Page column 64
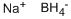
16940-66-2
8 suppliers
$21.21/100ml
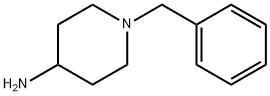
50541-93-0
366 suppliers
$6.00/5g
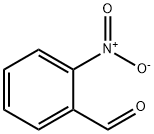
552-89-6
614 suppliers
$5.00/1g
![1-Benzyl-N-[(2-nitrophenyl)-methyl]piperidin-4-amine](/CAS/GIF/98754-28-0.gif)
98754-28-0
7 suppliers
inquiry