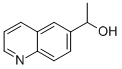
A-METHYL-6-QUINOLINEMETHANOL synthesis
- Product Name:A-METHYL-6-QUINOLINEMETHANOL
- CAS Number:880782-86-5
- Molecular formula:C11H11NO
- Molecular Weight:173.21
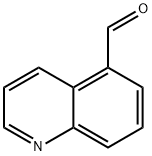
22934-41-4
125 suppliers
$44.00/100mg
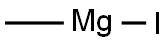
917-64-6
148 suppliers
$45.00/10ml

880782-86-5
14 suppliers
$60.00/50mg
Yield:880782-86-5 97%
Reaction Conditions:
Stage #1: quinoline-5-carbaldehyde;methyl magnesium iodide in tetrahydrofuran;diethyl ether at 0; for 2 h;
Stage #2: with water in tetrahydrofuran;diethyl ether;
Steps:
E.E
A solution of quinoline-5-carbaldehyde (available from Lancaster or Rare Chemicals GmbH) (4.65 g, 29.5 mmol) in THF (250 mL) was treated with MeMgI (11.8 mL, 35.4 mmol of a 3M solution in ether) in a dropwise fashion at 0° C. After 2 h the mixture was quenched with water, diluted with ethyl acetate, filtered through celite and separated into two layers. The aqueous layer was extracted with ethyl acetate/hexane. The organic fractions were pooled, dried over MgSO4, filtered, and evaporated to dryness. The alcohol, 1-quinolin-5-yl-ethanol was used without further purification, 5.0 g (97%). The alcohol, 1-quinolin-5-yl-ethanol (5.0 g, 28.9 mmol) in chloroform was treated with a dropwise addition of thionyl chloride (5.26 mL, 72.1 mmol) at rt. After 1 h the mixture was cooled to 0° C. and carefully quenched with a sat. solution of NaHCO3 followed by 2M NaOH until the pH was >8. The aqueous layer was extracted with chloroform and the organic fractions were combined, dried over MgSO4, filtered, and freed of solvent. The residue was purified by chromatography on SiO2 eluting with 30% ethyl acetate:hexane. The product, 5-(1-chloro-ethyl)-quinoline (Intermediate E1) was obtained as a clear oil, 4.22 g (76%). A solution of N-(diphenylmethylene)aminoacetonitrile (5.84 g, 26.0 mmol, commercially available from Aldrich) in THF (20 mL) and hexamethylphosphoramide (HMPA) (5.43 mL, 31 mmol) at -78° C. was reacted with lithium diisopropylamide (LDA) (15.4 mL of a 2M soln in heptane/THF/ethylbenzene) (commercially available from Aldrich). After 1 h, 5-(1-chloro-ethyl)-quinoline (Intermediate E1) (4.15 g, 21.7 mmol) in THF (15 mL) was introduced by dropwise addition. The mixture was kept at -78° C. for 5 m before removal of the cold bath. After 5 m, the mixture was quenched with cold water and extracted with ethyl acetate (3×). The organic solution was dried over MgSO4, filtered and concentrated to give 2-(benzhydrylidene-amino)-3-quinolin-5-yl-butyronitrile that was used in the next step without further purification. 2-(Benzhydrylidene-amino)-3-quinolin-5-yl-butyronitrile (8.36 g, 22.3 mmol) in dioxane (90 mL) was hydrolyzed with 1M HCl (90 mL) and the solution was stirred at rt for 16 h. The dioxane was removed under vacuum and the mixture was made basic with 2 M NaOH. The aqueous solution was extracted with chloroform:isopropanol (3:1). The organic extracts were pooled, dried over MgSO4, filtered and evaporated to leave an oil. The oily residue was purified by chromatography on SiO2, eluting with ethyl acetate and 5% methanol:ethyl acetate to give 2-amino-3-quinolin-5-yl-butyronitrile (Intermediate-E2) as a yellow solid 3.61 g (77%). In a Parr bottle, a mixture of 2-amino-3-quinolin-5-yl-butyronitrile (Intermediate-E2) (5.38 g, 25.4 mmol) in MeOH (100 mL) and ethylene diamine (3.2 mL, 47.8 mmol) was reacted with Raney 2800 nickel (18.9 g) and bubbled with ammonia gas for 10 m. The Parr bottle was pressurized with hydrogen at 50 psi and shaken on a Parr apparatus for 2.5 h at rt. The mixture was filtered through celite, washed with MeOH (3×) and evaporated to leave a residue. This material was placed onto a column and eluted with a gradient of 1% to 5% sat. NH3-MeOH:CH2Cl2 to give 3-quinolin-5-yl-butane-1,2-diamine (Intermediate E3) 3.9 g (71%). 3-Quinolin-5-yl-butane-1,2-diamine (Intermediate E3) (1.8 g, 8.35 mmol) in CH2Cl2 (20 mL) was treated with a solution of 1,1'-thiocarbonyldiimidazole (1.52 g, 8.54 mmol) in CH2Cl2 (40 L1) at 0° C. for 1 h. The mixture was diluted with chloroform (40 mL) and water (60 mL). The aqueous layer was extracted with chloroform (3×30 mL) and CH2Cl2 (6×30 mL). The organic solution was dried over MgSO4, filtered and evaporated to give a residue. The material was purified by chromatography on SiO2 with 3% NH3-MeOH:CH2Cl2 to give 4-(1-quinolin-5-yl-ethyl)-imidazolidine-2-thione (Intermediate E4) 2 g (93%). A mixture of 4-(1-quinolin-5-yl-ethyl)-imidazolidine-2-thione (Intermediate E4) (4.0 g, 15.5 mmol) in ethanol (120 mL) was treated with p-methoxybenzylchloride (4.22 mL, 27 mmol). The solution was heated in an oil bath (bath temp. 100° C.) for 1 h. The solvent was removed under vacuum and replaced with 10% NaOH (160 mL). The aqueous mixture was extracted with CH2Cl2 (2×100 mL) and chloroform (2×100 mL). The organic solution was dried over MgSO4, filtered, evaporated and purified by chromatography on SiO2 eluting with 2 to 4% NH3-MeOH:CH2Cl2. The product was 5-{1-[2-(4-methoxy-benzylsulfanyl)-4,5-dihydro-3H-imidazol-4-yl]-ethyl}-quinoline (Intermediate E5) 4.16 g (71%). A Swern-type reagent was formed in standard fashion: oxalyl chloride (9.15 mL as a 2M soln. in CH2Cl2) in CH2Cl2 (23 mL) was cooled to -78° C. and treated with a solution of DMSO (2.85 mL, 36.8 mmol) in CH2Cl2 (23 mL) for 30 m. To this solution was added 5-{-[2-(4-methoxy-benzylsulfanyl)-4,5-dihydro-3H-imidazol-4-yl]-ethyl}-quinoline (Intermediate E5) (4.7 g, 12.4 mmol) in CH2Cl2 (30 mL) and stirring continued for 45 m at -78° C. Triethylamine (9 mL) was added at -78° C. and warmed to rt for 40 m. The reaction mixture was diluted with brine and CH2Cl2. The aqueous layer was extracted with ethyl acetate. The organic solution was dried over MgSO4, filtered and evaporated to dryness. Chromatography on SiO2 with 80 to 100% ethyl acetate:hexanes yielded 5-{1-[2-(4-methoxy-benzylsulfanyl)-3H-imidazol-4-yl]-ethyl}-quinoline (Intermediate E6) 3.45 g (74%). 5-{1-[2-(4-Methoxy-benzylsulfanyl)-3H-imidazol-4-yl]-ethyl}-quinoline (Intermediate E6) (3 g, 8.0 mmol) and trifluoroacetic acid (100 mL) in a resealable tube was heated to 115° C. for 1 h 20 m. The mixture was cooled to rt and TFA removed under vacuum. The residual acid was quenched with NH3-MeOH. The residue was evaporated and resolvated with 3:1 chloroform:isopropanol (500 mL). The organic solution was washed with water (3×40 mL) and brine (1×30 mL). The solution was dried over MgSO4, filtered, and evaporated to leave a solid. This material was purified on a short column of silica gel by gradient elution with 3 to 9% NH3-MeOH:CH2Cl2 to give 4-(1-quinolin-5-yl-ethyl)-1,3-dihydro-imidazole-2-thione (Compound 7) 1.44 g (71%). 1H NMR (300 MHz, methanol-d4): δ 8.85 (dd, J=4.2, 1.5 Hz, 1H), 8.67 (d, J=8.4 Hz, 1H), 7.95 (d, J=9.0 Hz, 1H), 7.73 (dd, J=8.4, 7.2 Hz, 1H), 7.58 (dd, J=8.7, 4.5 Hz, 1H), 7.42 (d, J=6.6 Hz, 1H), 6.62 (s, 1H), 4.80-4.85 (m, 1H), 1.67 (d, J=7.2 Hz, 3H).
References:
US2006/69144,2006,A1 Location in patent:Page/Page column 23-25
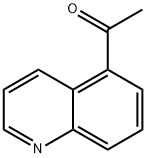
103854-56-4
32 suppliers
inquiry

880782-86-5
14 suppliers
$60.00/50mg

22934-41-4
125 suppliers
$44.00/100mg
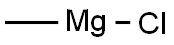
676-58-4
286 suppliers
$15.00/25ml

880782-86-5
14 suppliers
$60.00/50mg

4964-71-0
305 suppliers
$6.00/1g

880782-86-5
14 suppliers
$60.00/50mg

22934-41-4
125 suppliers
$44.00/100mg

75-16-1
270 suppliers
$12.00/10ml

880782-86-5
14 suppliers
$60.00/50mg