ABT-199 synthesis
- Product Name:ABT-199
- CAS Number:1257044-40-8
- Molecular formula:C45H50ClN7O7S
- Molecular Weight:868.44

Reference: Ku, Yi-Yin; Chan, Vincent S.; Christesen, Alan; Grieme, Timothy; Mulhern, Mathew; Pu, Yu-Ming; Wendt, Michael D. Development of a Convergent Large-Scale Synthesis for Venetoclax, a First-in-Class BCL-2 Selective Inhibitor. Journal of Organic Chemistry. Volume 84. Issue 8. Pages 4814-4829. Journal; Online Computer File. (2019).
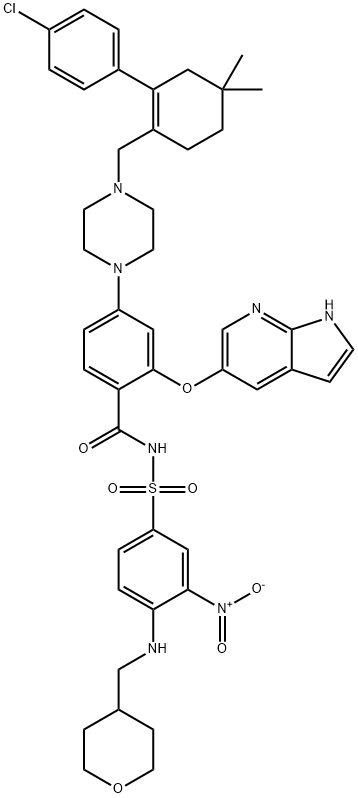
1228779-96-1
203 suppliers
$5.00/250mg
![2-((1H-Pyrrolo[2,3-b]pyridin-5-yl)oxy)-4-(4-((4'-chloro-5,5-dimethyl-3,4,5,6-tetrahydro-[1,1'-biphenyl]-2-yl)methyl)piperazin-1-yl)benzoic acid](/CAS/20180713/GIF/1235865-77-6.gif)
1235865-77-6
104 suppliers
inquiry

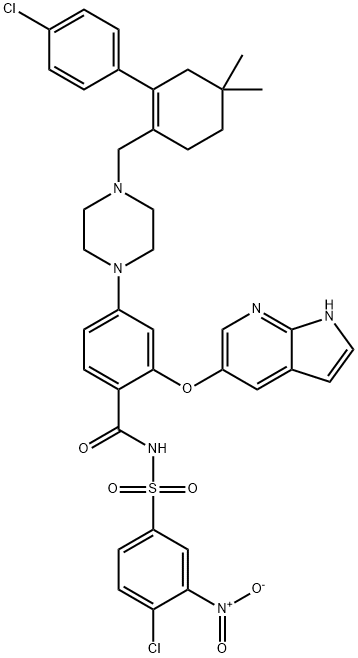
1257044-40-8
461 suppliers
$12.00/1mg
Yield:1257044-40-8 91.4%
Reaction Conditions:
with dmap;1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride;triethylamine in dichloromethane at 20; for 3 h;Reagent/catalyst;Temperature;
Steps:
2 Synthesis of Compound I
Compound IX 20.3 g (0.064 mol), 15.8 g (0.13 mol) DMAP, 20.9 g were added to a 2 L reaction flask.(0.11 mol) EDCI and 500 mL dichloromethane (DCM),The system was stirred at 20 °C.A solution of 37.5 g (0.064 mol) of compound VIII and 23.7 g (0.23 mol) of triethylamine and 200 mL of dichloromethane prepared in advance was slowly added dropwise to the above system.After the completion of the dropwise addition, the mixture was stirred at 20 ° C for 3 hours, and then controlled by HPLC or TLC. The starting material was completely reacted.To the system was added 14.1 g of N,N'-dimethylethylenediamine.The system is heated to 35 ° C and stirring is continued.After the temperature is stable, add 200 mL of 12% acetic acid solution.After stirring for 10 min, the layers were separated, and the organic layer was washed with 5% sodium hydrogen carbonate solution (200 mL) and then washed with 5% sodium chloride solution (200 mL).The organic layer was dried, filtered, and evaporated to dryness.After the system is heated to 30 ° C, the methanol solution is added dropwise.After the system is clarified, start to cool down to 0-5 ° C, and stir for 1 h.After filtration, the cake was dried to give a white solid compound I about 50.8 g.The yield is 91.4%,
References:
CN108997333,2018,A Location in patent:Paragraph 0065; 0067; 0075; 0084; 0094; 0103; 0113
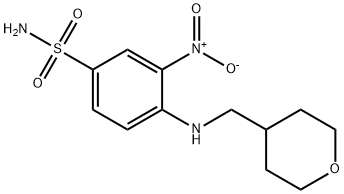
130290-79-8
304 suppliers
$6.00/1g
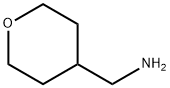
1257044-99-7
5 suppliers
inquiry

1257044-40-8
461 suppliers
$12.00/1mg
![Methyl 4,4-dimethyl-2-[(trifluoromethylsulfonyl)oxy]cyclohex-1-ene-1-carboxylate](/CAS/20180703/GIF/1228780-46-8.gif)
1228780-46-8
6 suppliers
inquiry

1257044-40-8
461 suppliers
$12.00/1mg
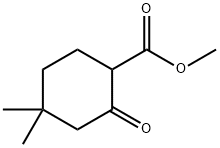
32767-46-7
34 suppliers
$120.00/50mg

1257044-40-8
461 suppliers
$12.00/1mg

