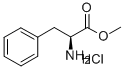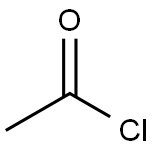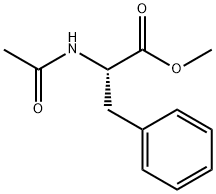
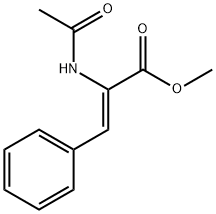
AC-PHE-OME synthesis
- Product Name:AC-PHE-OME
- CAS Number:3618-96-0
- Molecular formula:C12H15NO3
- Molecular Weight:221.25
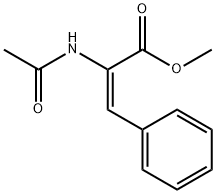
64590-81-4
0 suppliers
inquiry

3618-96-0
90 suppliers
$10.00/1g
Yield:3618-96-0 100%
Reaction Conditions:
with hydrogen;bis(norbornadiene)rhodium(l)tetrafluoroborate;(S)Fc-α-S(C5H5)Fe(C5H3(PPh2)CH(OH)C6H4(PPh2)) in methanol at 25; under 787.579 Torr; for 1 h;Product distribution / selectivity;
Steps:
D.1
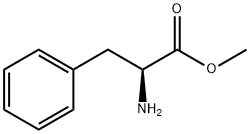
0.555 g (2.53 mmol) of methyl trans-acetamidocinnamate and 5 ml of degassed methanol are introduced in succession into a Schlenk vessel filled with argon. A catalyst solution consisting of 4.73 g (0.01265 mmol) of [Rh(norbomadiene)2]BF4, 8.77 mg (0.0133 mmol) of ligand A and 5 ml of degassed methanol is prepared in a second Schlenk vessel filled with argon. This solution and the catalyst solution are then transferred in succession by means of a steel capillary into a 50 ml glass reactor filled with argon. The ratio of substrate/catalyst (s/c) is 200. The reactor is closed and a pressure of 1.05 bar is set by means of 4 flushing cycles (pressurization to 1 bar of hydrogen). The autoclave is thermostated at 25°C and the reaction is started by switching on the stirrer. The reactor is stirred for 1 hour. After opening the reactor, a reddish reaction solution is isolated. The conversion is quantitative (determined by means of GC and 1H-NMR). Removal of the solvent on a rotary evaporator gives a quantitative yield of the methyl ester of (S)-N-acetylphenylalanine having an enantiomeric purity of 99.1% ee (determined by means of GC; column: Chirasil-L-Val.).
References:
WO2005/108409,2005,A2 Location in patent:Page/Page column 30
60676-51-9
51 suppliers
$38.00/250mg

3618-96-0
90 suppliers
$10.00/1g
2577-90-4
87 suppliers
$102.00/1g
108-24-7
5 suppliers
$14.00/250ML

3618-96-0
90 suppliers
$10.00/1g