AC-P-AMINO-PHE-OME synthesis
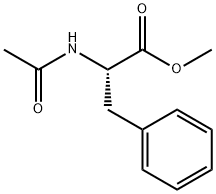
- Product Name:AC-P-AMINO-PHE-OME
- CAS Number:36097-42-4
- Molecular formula:C12H16N2O3
- Molecular Weight:236.27

36097-39-9
0 suppliers
inquiry

36097-42-4
19 suppliers
inquiry
Yield:36097-42-4 75.2%
Reaction Conditions:
with iron(0);ammonia hydrochloride;glacial acetic acid in ethanol;lithium hydroxide monohydrate at 90; for 3 h;
Steps:
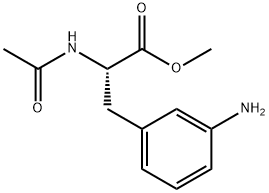
1.4 (4) Preparation of (S)-methyl 2-acetamido-3-(4-aminophenyl) propionate (ie, a compound A5)
Reduced iron powder (11.1g, 0.199mol), ammonium chloride (0.68g, 0.013mol),Ethanol (56mL), water (14mL) and glacial acetic acid (3.4mL) were sequentially added to a 250mL three-necked flask equipped with a drying tube device, heated to 90°C for reflux reaction, mechanically stirred for 1.5h, (S)-Methyl 2-acetamido-3-(4-nitrophenyl) propionate (5.1 g, 0.019 mol) was added to continue the reaction for 3 h, suction filtered while hot, the filtrate was concentrated under reduced pressure, and dichloromethane ( 150mL), extract, add water (50mL×2) to wash, dry the organic phase over anhydrous magnesium sulfate for 4h; filter, and concentrate the filtrate under reduced pressure,3.4 g of yellow-white solid were obtained, yield: 75.2%.
References:
CN113845485,2021,A Location in patent:Paragraph 0048; 0059-0061

17363-92-7
22 suppliers
inquiry

36097-42-4
19 suppliers
inquiry
2018-61-3
375 suppliers
$14.00/5G

36097-42-4
19 suppliers
inquiry