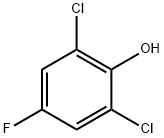
Acetic acid, 2-(2,6-dichloro-4-fluorophenoxy)- synthesis
- Product Name:Acetic acid, 2-(2,6-dichloro-4-fluorophenoxy)-
- CAS Number:392-17-6
- Molecular formula:C8H5Cl2FO3
- Molecular Weight:239.03
Yield:392-17-6 72.8%
Reaction Conditions:
Stage #1: 2,6-dichloro-4-fluorophenol;ethyl bromoacetatewith potassium carbonate in acetone; for 12 h;Heating / reflux;
Stage #2: with sodium hydroxide in 1,4-dioxane;water at 20;
Stage #3: with hydrogenchloride in 1,4-dioxane;water;
Steps:
21.A
The mixture of 2,6-dichloro-4-fluorophenol (362 mg, 2.0 mmol), potassium carbonate (910 mg, 6.6 mmol), ethyl bromoacetate (500 mg, 3.0 mmol) and acetone (25 ml) was refluxed for 12 h. After cooling, the mixture was filtered to remove potassium carbonate. The filtrate was concentrated under reduced pressure. To this residue, 10 ml dioxane and 14 ml 5% sodium hydroxide solution were added. After the mixture was stirred at room temperature overnight, it was acidified with concentrated hydrochloric acid to pH 2, and then extracted three times with ethyl acetate (15 ml each). Organic phases were combined, washed with water and brine, dried over magnesium sulfate, filtered, and then evaporated in vacuo. The residue was recrystallized from ethyl acetate and petroleum ether to give 348 mg 2,6-dichloro-4-fluorophenoxyacetic acid as white crystals, mp 155-158° C., yield: 72.8%. The chemical structure analysis was performed by 1HNMR (Acetone-d6, 600 MHz): δ 7.36 (m, 2H, Ar-H), 4.67 (s, 2H, OCH2CO).
References:
US6350756,2002,B1 Location in patent:Page column 43

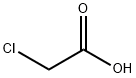
371-41-5
505 suppliers
$8.00/25g

392-17-6
1 suppliers
inquiry
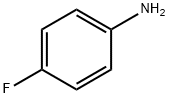
371-40-4
403 suppliers
$5.00/5G

392-17-6
1 suppliers
inquiry
392-71-2
114 suppliers
$17.00/1g

392-17-6
1 suppliers
inquiry