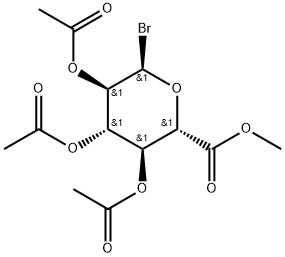
ACETOBROMO-ALPHA-D-GLUCURONIC ACID METHYL ESTER synthesis
- Product Name:ACETOBROMO-ALPHA-D-GLUCURONIC ACID METHYL ESTER
- CAS Number:21085-72-3
- Molecular formula:C13H17BrO9
- Molecular Weight:397.17
7355-18-2
230 suppliers
$6.00/1g

21085-72-3
224 suppliers
$11.00/1g
Yield:21085-72-3 96.98%
Reaction Conditions:
with hydrogen bromide;acetic acid at 0 - 20; for 2 h;
Steps:
3-O-acetyl-α-D-bromo-glucuronic acid methyl ester (9)
Compound 8 (5 g, 0.0133 mol) was slowly added to 33 % acetic acid solution of hydrogen bromide (20 mL) at 0 °C. The mixture was stirred at room temperature for 2 h. Benzene (50 mL) was added to the mixture, and the resulting mixture was concentrated under reduced pressure to syrup. The syrup was dissolved with ethyl acetate (100 mL). Then the mixture was washed with saturated sodium bicarbonate, dried over anhydrous sodium sulfate, filtered and concentrated under reduced pressure to afford 9 (5.12 g, 96.98%). mp 102.5~103.8 °C; ESI-MS (m/z): Cacld for {[C13H18BrO9]+} 397.0134/399.0114, found 396.9410/398.9388. 1H NMR(300 MHz, CDCl3) δ: 5.79, 5.77 (1H, d, -CH), 5.34~5.13 (3H, m, 4×-CH), 4.21, 4.18 (1H, d, -CH), 3.75 (3H, s, -OCH3), 2.05~2.04 (9H, m, 3×-COCH3); 13C NMR (75 MHz, CDCl3) δ: 169.80, 169.03, 168.45, 167.88 (4×-COCH3), 89.16 (-CH), 72.95, 71.42, 70.22, 69.89 (4×-CH), 52.79 (-OCH3), 20.45, 20.41, 20.38 (3×-COCH3).
References:
Zhu, Hao-Hao;Chen, Yu-Qing;Cheng, Dong;Li, Wei;Wang, Tian-Lin;Wen, Hong-Mei;Chen, Long;Liu, Jian [Bioorganic and Medicinal Chemistry Letters,2016,vol. 26,# 3,p. 882 - 884] Location in patent:supporting information

108-24-7
5 suppliers
$14.00/250ML
32449-92-6
468 suppliers
$13.43/1gm:

21085-72-3
224 suppliers
$11.00/1g
5432-32-6
38 suppliers
$463.00/1g

21085-72-3
224 suppliers
$11.00/1g

67-56-1
738 suppliers
$7.30/5ml-f

124-41-4
686 suppliers
$12.00/25g

108-24-7
5 suppliers
$14.00/250ML

64-19-7
1539 suppliers
$10.00/25ML

32449-92-6
468 suppliers
$13.43/1gm:

21085-72-3
224 suppliers
$11.00/1g
