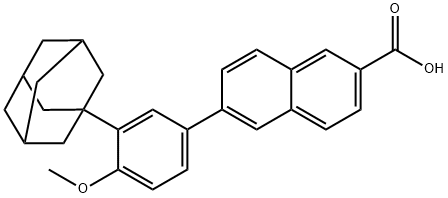
Adapalene synthesis
- Product Name:Adapalene
- CAS Number:106685-40-9
- Molecular formula:C28H28O3
- Molecular Weight:412.52
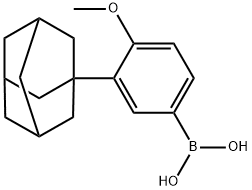
459423-32-6
89 suppliers
$50.00/25mg
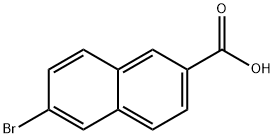
5773-80-8
302 suppliers
$10.00/1g

106685-40-9
597 suppliers
$6.00/50mg
Yield:106685-40-9 99%
Reaction Conditions:
Stage #1: 3-(1-adamantyl)-4-methoxyphenylboronic acid;6-bromo-2-naphthoic acidwith potassium carbonate;palladium 10% on activated carbon in tetrahydrofuran;water; for 8 h;Heating / reflux;
Stage #2: with hydrogenchloride;water in tetrahydrofuran; for 1 h;Product distribution / selectivity;
Steps:
3
Example 3 :Preparation of 6- [3- (1-adamantyl) -4-methoxyphenyl] -2- naphthoic acid (I) :20 ml (12 vol) of tetrahydrofuran, 2 g (7 mmol) of 3-adamantyl-4-methoxyphenylboronic acid (II), 1.65 g (6.6 mmol) of 6 -broτno-2 -naphthoic acid (III) and 20 mL of a 2 M aqueous potassium carbonate solution are introduced into a round-bottomed flask equipped with EPO
References:
WO2006/108717,2006,A2 Location in patent:Page/Page column 11-12