Adefovir synthesis
- Product Name:Adefovir
- CAS Number:106941-25-7
- Molecular formula:C8H12N5O4P
- Molecular Weight:273.19
![[[2-(6-Amino-9H-purin-9-yl)ethoxy]methyl]phosphonic acid diethyl ester](/CAS/GIF/116384-53-3.gif)
116384-53-3
174 suppliers
$27.00/1g

106941-25-7
334 suppliers
$5.00/250mg
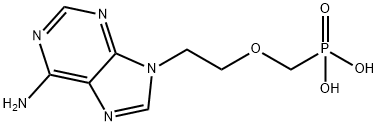
Yield:106941-25-7 86.3%
Reaction Conditions:
Stage #1:[[2-(6-amino-9H-purine-9-yl)ethoxy]methyl]phosphonic acid diethyl ester with trimethylsilyl bromide in acetonitrile at 20; for 3 h;Large scale;
Stage #2: with sodium hydroxide in water for 2 h;Reflux;Large scale;
Steps:
4 Preparation of 9-(2-phosphomethoxyethyl) adenine (Intermediate IV, PMEA)
A mechanical stirrer and a thermometer were installed in a 10 L three-necked flask. To this was added 1.4 kg (4.26 mol) of intermediate III, Trimethylsilyl bromide in this orderAnd 8 L of acetonitrile were added thereto. The mixture was stirred at room temperature for 1 hour and heated to reflux for 2 hours. After completion of the reaction, the solvent was distilled off under reduced pressure, 5 L of water was added, and the mixture was stirred and adjusted to pH 3.2 to 3.4 with 25% sodium hydroxide. The mixture was heated to reflux for 2 hours. After completion of the reaction, the reaction mixture was cooled to room temperature and filtered. The cake was recrystallized once from water and vacuum dried at 70 ° C for 8 hours. 9- (2-phosphomethoxyethyl) adenine as a white crystalline solid powder 1.07kg, Μρ: 298 ~ 300 ° (3, yield 86.3%.
References:
Suzhou Erye Pharmaceutical Co., Ltd.;ZHANG, JIAN;ZHU, WEI;YAO, CHANYAN;CHEN, XUEWEN CN104387421, 2016, B Location in patent:Paragraph 0032-0034
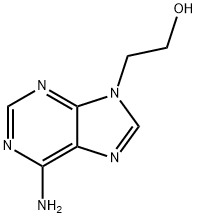
707-99-3
133 suppliers
$15.00/1g

106941-25-7
334 suppliers
$5.00/250mg
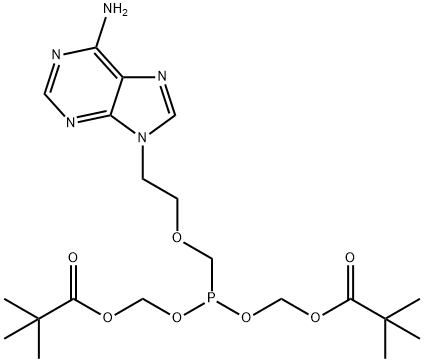
142340-99-6
464 suppliers
$5.00/10mg

106941-25-7
334 suppliers
$5.00/250mg

1670-62-8
5 suppliers
inquiry

106941-25-7
334 suppliers
$5.00/250mg

56791-59-4
2 suppliers
inquiry

106941-25-7
334 suppliers
$5.00/250mg