AKOS 91083 synthesis
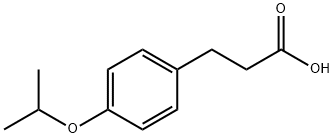
- Product Name:AKOS 91083
- CAS Number:79669-11-7
- Molecular formula:C12H16O3
- Molecular Weight:208.25
586960-22-7
15 suppliers
$90.00/500mg

79669-11-7
13 suppliers
$140.00/500mg
Yield:-
Reaction Conditions:
with hydrogen;palladium 10% on activated carbon in ethanol;ethyl acetate; under 4121.6 Torr; for 16 h;
Steps:
10
A 125 mL Ehrlenmayer flask equipped with a magnetic stirrer bar was charged with 7.76 g (47.8 mmol) of 4-Isopropoxybenzaldehyde, 5.28 g (50.7 mmol) of malonic acid and 2.2 mL (2.2g, 27.2 mmol) of pyridine. The slurry was heated to 800C, at which temperature a clear yellow solution formed. After stirring for 2 hr, the reaction mixture was cooled to 25°C. The resulting solid was isolated by filtration, rinsing with water (2 X 30 mL) and 2: 1 methyl t-butyl ether (MTBE)/hexanes (2 X 30 mL). A total of 3.1 g of 4-Isopropoxycinnamic acid was isolated.
References:
WO2008/112368,2008,A2 Location in patent:Page/Page column 32-33
![Propanedioic acid, 2-[[4-(1-methylethoxy)phenyl]methyl]-, 1,3-dimethyl ester](/CAS/20210305/GIF/365572-34-5.gif)
365572-34-5
0 suppliers
inquiry

79669-11-7
13 suppliers
$140.00/500mg
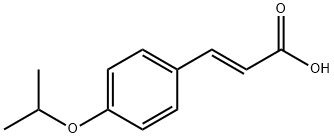
![2-Propenoicacid, 3-[4-(1-methylethoxy)phenyl]-](/CAS/20180601/GIF/20718-97-2.gif)
20718-97-2
3 suppliers
inquiry

79669-11-7
13 suppliers
$140.00/500mg
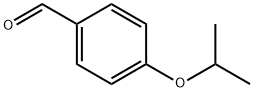
18962-05-5
181 suppliers
$6.00/1g

79669-11-7
13 suppliers
$140.00/500mg