
AKOS B023083 synthesis
- Product Name:AKOS B023083
- CAS Number:161119-99-9
- Molecular formula:C8H13NO
- Molecular Weight:139.19
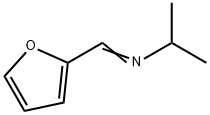
65443-11-0
0 suppliers
inquiry

161119-99-9
12 suppliers
$65.00/50mg
Yield:-
Reaction Conditions:
with sodium tetrahydroborate in methanol;dichloromethane at 5 - 20; for 24 h;
Steps:
Synthesis of N-furfuryl-N-isopropylamine
Isopropylamine (0.4 ml, 4 mmol) and furfuraldehyde (0.4 ml, 5.1 mmol) were dissolved inmethanol (10 ml) and the solution was stirred for 2 h at room temperature. The solvent wasremoved by evaporation. The resulting yellow oil was dissolved in methanol-dichloromethanesolvent mixture (1:1, 20 ml) and sodium borohydride (0.5 g, 13.8 mmol) was added slowly at5C. The mixture was stirred at room temperature for 24 h. After evaporation of the solvent, theresulting viscous liquid was washed with water and dichloromethane was added in order to extractthe product. Evaporation of the organic layer gave N-furfuryl-N-isopropylamine as yellow oil.1H NMR (400 MHz, CDCl3, ppm): δ = 1.07 (d, 6H, J = 6.0 Hz, methyl protons), 2.06 (b, 1H,NH), 2.78-2.84 (m, 1H, methine proton), 4.82 [s, 2H, NCH2(furfuryl)], 6.16 [b, 1H, H-4 (furyl)],6.30 [dd, 1H, J = 1.2 Hz, H-3 (furyl)], 7.34 [d, 1H, J = 0.8 Hz, H-5 (furyl)]. 13CNMR(100 MHz,CDCl3, ppm): δ = 22.7 (methyl carbons), 43.8 (methine carbons), 47.6 [NCH2(furfuryl)], 106.6,110.1, 141.7, 154.1(furyl carbons).
References:
Rani, Palanisamy Jamuna;Thirumaran, Subbiah;Ciattini, Samuele [Journal of Sulfur Chemistry,2014,vol. 35,# 1,p. 106 - 116]
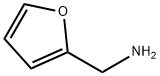
617-89-0
264 suppliers
$16.00/25g

67-64-1
6 suppliers
$19.10/10ml

161119-99-9
12 suppliers
$65.00/50mg
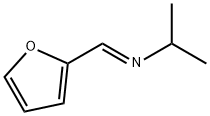
104727-72-2
0 suppliers
inquiry

161119-99-9
12 suppliers
$65.00/50mg

617-89-0
264 suppliers
$16.00/25g

161119-99-9
12 suppliers
$65.00/50mg
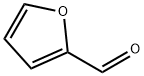
98-01-1
480 suppliers
$22.00/25g

161119-99-9
12 suppliers
$65.00/50mg