
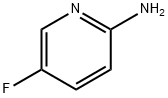
(5-Fluoro-pyridin-2-yl)-Methyl-aMine synthesis
- Product Name:(5-Fluoro-pyridin-2-yl)-Methyl-aMine
- CAS Number:868636-72-0
- Molecular formula:C6H7FN2
- Molecular Weight:126.13

74-88-4
340 suppliers
$15.00/10g

868636-72-0
42 suppliers
$110.00/100mg
Yield:868636-72-0 23%
Reaction Conditions:
Stage #1: 2-amino-5-fluoropyridinewith sodium hydride in tetrahydrofuran at 40; for 0.5 h;
Stage #2: methyl iodide in tetrahydrofuran at -40 - 20;
Steps:
25.1
STEP 1. 5-Fluoro-N-methylpyridin-2-amine; To a suspension of sodium hydride (60% dispersion in mineral oil, 117.8 mg, 4.91 mmol) in tetrahydrofuran (25 ml) was added a solution of 5-fluoropyridin-2-amine (500 mg, 4.46 mmol) in tetrahydrofuran (25 ml) at room temperature and the reaction mixture was stirred at 40 °C for 30 min. Then to the reaction mixture was added methyl iodide (696.9 mg, 4.91 mmol) at -40 °C and the resulting mixture was stand at room temperature overnight with stirring. The reaction was quenched by the addition of water and whole mixture was extracted with ethyl acetate. The organic extracts were dried over sodium sulfate and concentrated. The residue was purified by flush column chromatography on silica gel eluting with hexane/ethyl acetate (4/1) to afford 129 mg (23%) of the title compound: 1H-NMR (CDCl3) No. 7.97 (1 H, d, J = 2.6 Hz), 7.28-7.17 (1 H, m), 6.34 (1 H, dd, J = 3.5, 9.1 Hz), 2.90 (3H, d, J = 5.1 Hz).
References:
WO2005/105732,2005,A1 Location in patent:Page/Page column 57-58
21717-96-4
409 suppliers
$10.00/1g

74-88-4
340 suppliers
$15.00/10g

868636-72-0
42 suppliers
$110.00/100mg