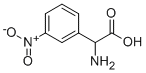
AMINO-(3-NITRO-PHENYL)-ACETIC ACID synthesis
- Product Name:AMINO-(3-NITRO-PHENYL)-ACETIC ACID
- CAS Number:30077-08-8
- Molecular formula:C8H8N2O4
- Molecular Weight:196.16
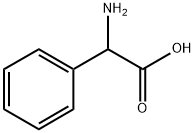
2835-06-5
281 suppliers
$6.00/25g

30077-08-8
34 suppliers
inquiry
Yield:30077-08-8 64%
Reaction Conditions:
with sulfuric acid;nitric acid at 0 - 20; for 1.5 h;
Steps:
Amino(3-nitrophenyl)acetic acid (1) (Sassatelli et al. 2006)
Amino(phenyl)acetic acid (33 mmol, 5 g) was cooled at 0 °C and added to a mixture of concentrated H2SO4 (98% w/w,5 mL) and fuming HNO3 (90% w/w, 5 mL). The mixturewas stirred for 1 h at 0 °C then 30 min at r.t. After this, thereaction was hydrolyzed with cold water (60 mL). The pHwas adjusted by addition of 10M NH4OH. The resultingmixture was stirred at 4 °C for 10 h, 3-nitrophenylglycinewas isolated by filtration. After washing with cold water,the compound was isolated as a yellow powder. Scheme S1.Yield: 64%. 1H-NMR (DMSO, 200 MHz): δ = 8.27 (s, H-4,1H), 8.10 (d, J = 8.1 Hz, H-6, 1H), 7.83 (d, J = 7.9 Hz, H-8,1H), 7.64 (t, J = 7.9 Hz, H-7, 1H), 4.51 (s, H-2, 1H). Anal.calcd. for C8H8N2O4: C, 48.98; H, 4.11; N, 14.28. Found:C, 48.92; H, 4.19; N, 14.31.
References:
Genovese, Filippo;Lazzari, Sandra;Venturi, Ettore;Costantino, Luca;Blazquez, Jesus;Ibacache-Quiroga, Claudia;Costi, Maria Paola;Tondi, Donatella [Medicinal Chemistry Research,2017,vol. 26,# 5,p. 975 - 986]
2935-35-5
384 suppliers
$5.00/10g

30077-08-8
34 suppliers
inquiry

4885-81-8
1 suppliers
inquiry

30077-08-8
34 suppliers
inquiry