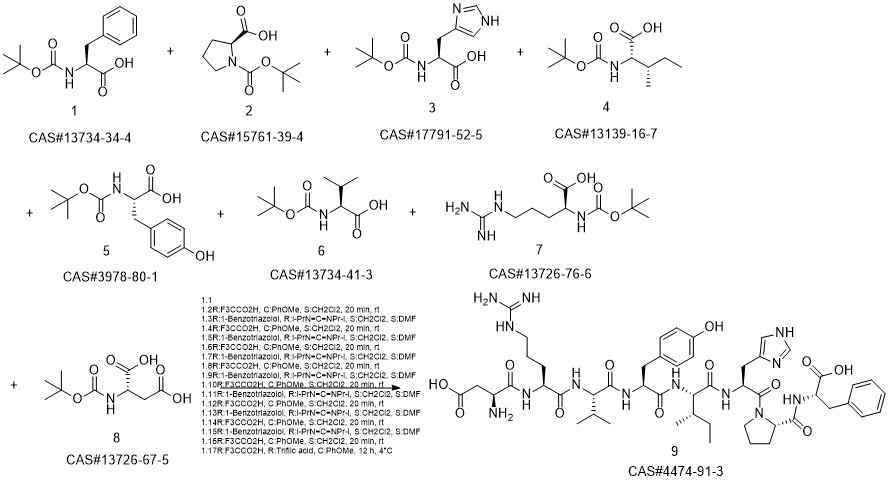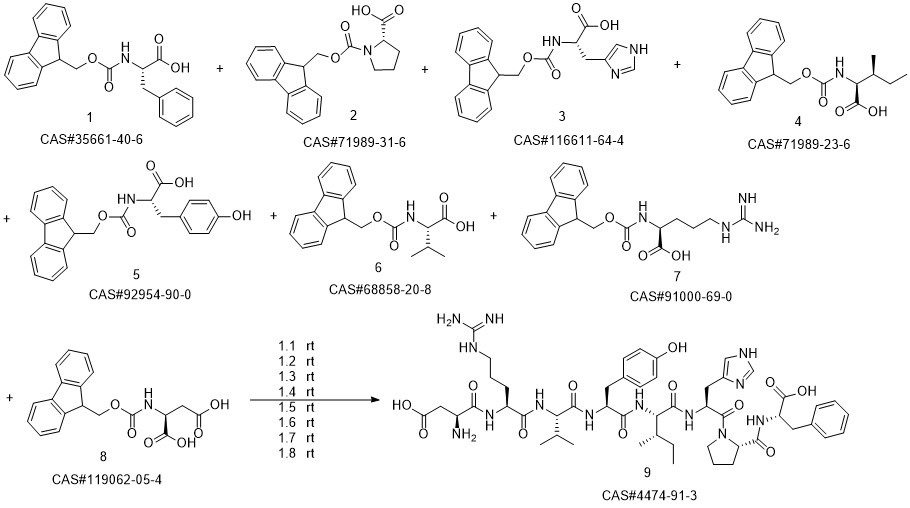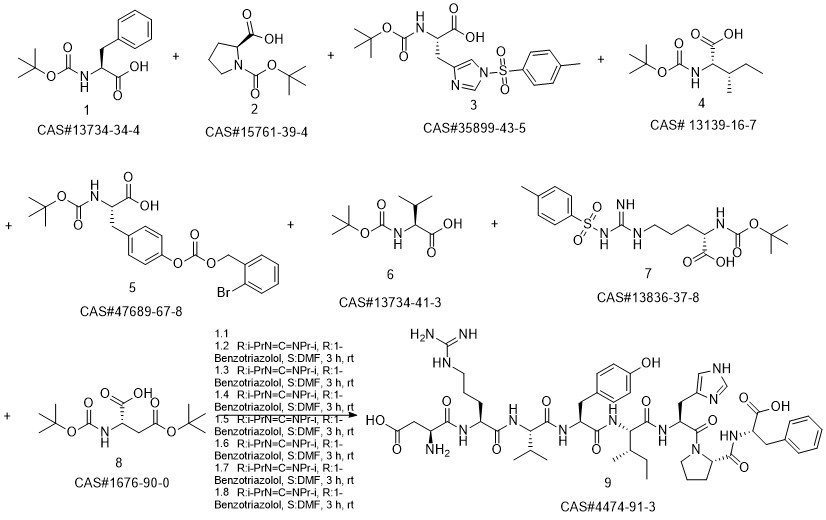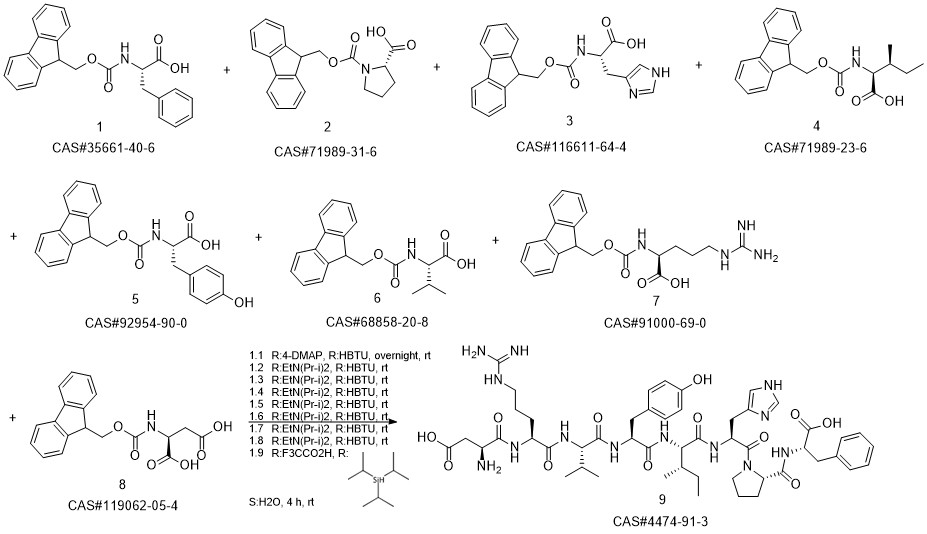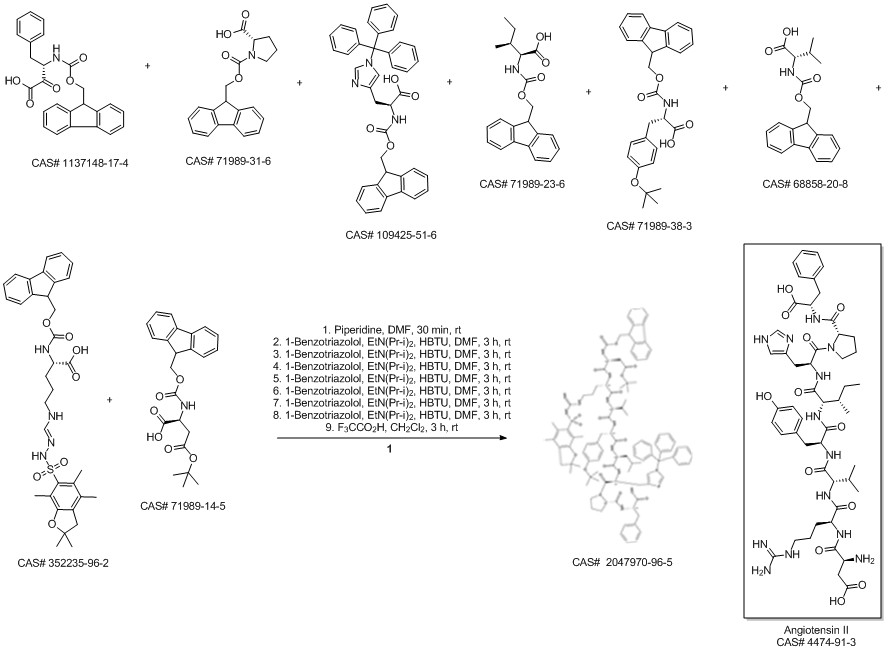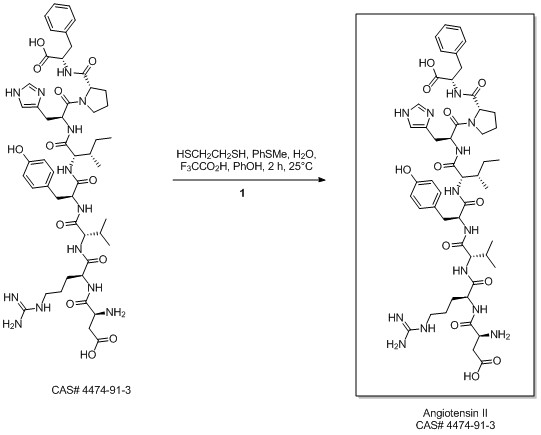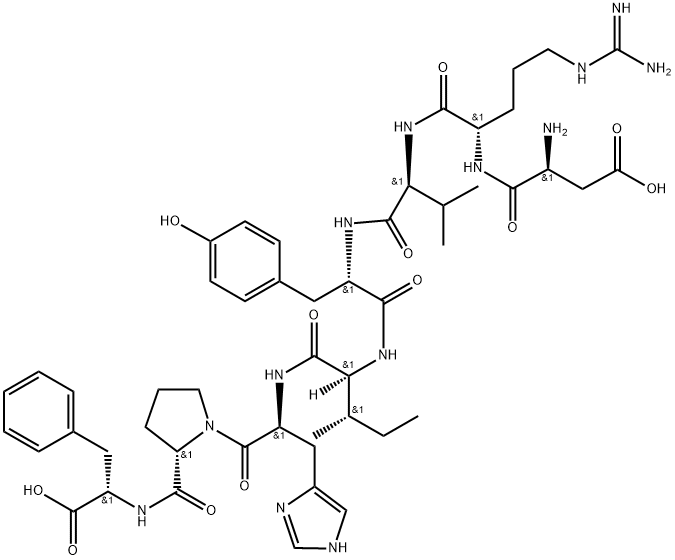
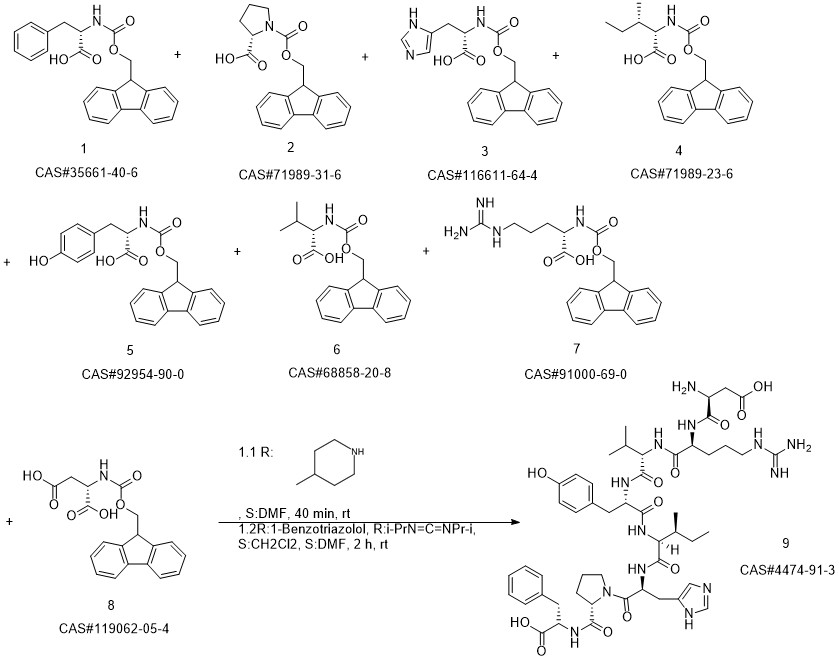
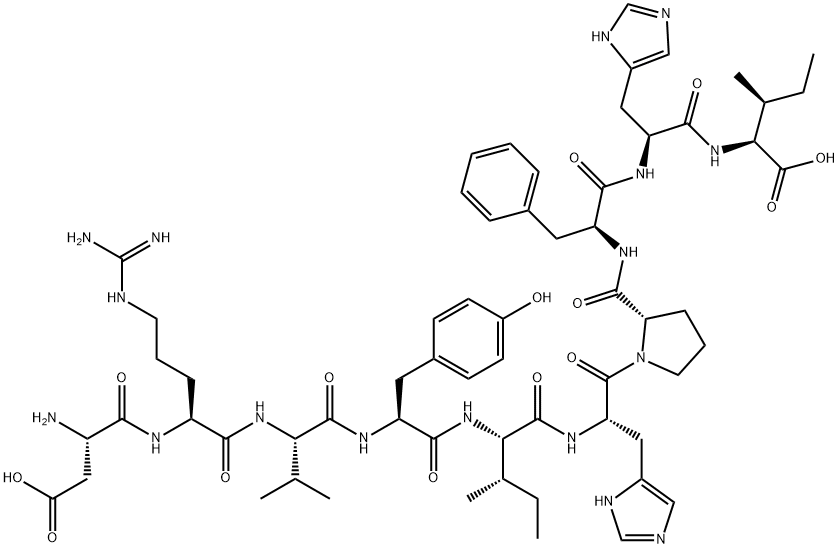
ANGIOTENSIN II, HUMAN synthesis
- Product Name:ANGIOTENSIN II, HUMAN
- CAS Number:4474-91-3
- Molecular formula:C50H71N13O12
- Molecular Weight:1046.2
Reference: Marcelo Der Torossian T, Silva AF, Alves FL, Capurro ML, Miranda A, Vani Xavier O Jr. Highly potential antiplasmodial restricted peptides. Chem Biol Drug Des. 2015 Feb;85(2):163-71. doi: 10.1111/cbdd.12354. Epub 2014 Jul 11. PubMed PMID: 24800635.
484-42-4
127 suppliers
$46.59/5MG

4474-91-3
218 suppliers
$19.00/1mg

7763-65-7
90 suppliers
$9.00/250mg
Yield:-
Reaction Conditions:
in water at 30; for 0.55 h;Shimeji kininase enzyme, 0.01 M pH 7.4 phosphate buffer;
References:
Kizuki;Moriya [Chemical and Pharmaceutical Bulletin,1981,vol. 29,# 9,p. 2721 - 2724]