BAY 11-7082 synthesis
- Product Name:BAY 11-7082
- CAS Number:19542-67-7
- Molecular formula:C10H9NO2S
- Molecular Weight:207.25

107-13-1
399 suppliers
$16.00/25ML

98-59-9
587 suppliers
$9.00/5g

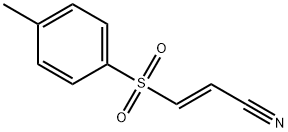
19542-67-7
165 suppliers
$24.00/5mg
Yield: 54%
Reaction Conditions:
Stage #1:acrylonitrile;p-toluenesulfonyl chloride with copper(l) iodide;triethylamine hydrochloride in sulfolane at 100; for 3 h;Large scale;
Stage #2: with triethylamine in water at 20;Large scale;
Steps:
2 Example 2: Synthesis of (E)-3-tosylacrylonitrile:
1.906 g (10.0 mmol) of tosyl chloride and 1.31 ml_ (2 eq.) of acrylonitrile was charged into the reaction vessel with 2.85 ml_ sulfolane; magnetic stirring was initiated and the oil bath was set to 100 °C. Solution of 0.206 g (0.15 eq.) of the catalyst (Cul-TEA*HCI) to the hot reaction mixture in one portion. Reaction mix- ture was stirred for 3h at 100 °C and monitored by HPLC. (0046) Reaction mixture was cooled to room temperature, poured into 1.4 ml_ of triethyl- amine (TEA) in 20 mL purified water. The precipitate that was formed was filtered off and washed with purified water (2 x 5 mL) and 0.3 M HCI (2 x 5 mL) and the collected brownish precipitate dried. Crude yield was 1.85 g of (E)-3-tosylacrylo- nitrile (89 %). (0047) The crude precipitate was sonicated in 50 mL of Diethyl ether and an insoluble precipitate was filtered off. The mother liquor was concentrated in vacuo to 1/3 volume (40 °C, P=650 torr) and product started to precipitate. The precipitate was filtered off and washed on filter with cold Diethyl ether (2 c 25mL) and then dried on a lyophilizer. The yield of purified (E)-3-tosylacrylonitrile as white crystals was 1.13 g (54 %). (0048) The identity and purity of the product was confirmed by NMR and HPLC-MS.
References:
KEMIRA OYJ;HILTUNEN, Jaakko;SUOMINEN, Petteri;OGIBALOV, Ivan;UUSTARE, Ain WO2020/94917, 2020, A1 Location in patent:Page/Page column 9
![Propanenitrile, 2-iodo-3-[(4-methylphenyl)sulfonyl]-](/CAS/20210305/GIF/19520-58-2.gif)
19520-58-2
0 suppliers
inquiry

19542-67-7
165 suppliers
$24.00/5mg
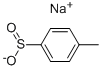
824-79-3
401 suppliers
$6.00/25g

107-13-1
399 suppliers
$16.00/25ML

19542-67-7
165 suppliers
$24.00/5mg

824-79-3
401 suppliers
$6.00/25g

107-13-1
399 suppliers
$16.00/25ML

19542-67-7
165 suppliers
$24.00/5mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

107-13-1
399 suppliers
$16.00/25ML
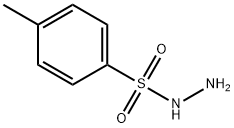
1576-35-8
451 suppliers
$10.00/1g

19542-67-7
165 suppliers
$24.00/5mg