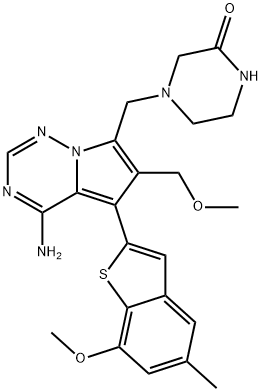
BAY-1163877 synthesis
- Product Name:BAY-1163877
- CAS Number:1443530-05-9
- Molecular formula:C23H26N6O3S
- Molecular Weight:466.56
![(7-Methoxy-5-Methylbenzo[b]thiophen-2-yl)boronic acid](/CAS/20150408/GIF/1443531-60-9.gif)
1443531-60-9
39 suppliers
$45.00/2.5mg
![2-Piperazinone, 4-[[4-amino-5-bromo-6-(methoxymethyl)pyrrolo[2,1-f][1,2,4]triazin-7-yl]methyl]-](/CAS/20210305/GIF/1443532-21-5.gif)
1443532-21-5
0 suppliers
inquiry

1443530-05-9
67 suppliers
inquiry
Yield: 78%
Reaction Conditions:
with dichloro[1,1′-bis[bis(1,1-dimethylethyl)phosphino]ferrocene-P,P′]palladium;potassium carbonate in tetrahydrofuran;water at 64; under 150.015 Torr; for 3.5 h;Inert atmosphere;Large scale;
Steps:
8 Example 8: 4-l l4-amino-6-(mnethoxymethyl)-5-(7-methoxy-5-methyl-l -benzothionhen-2- yl)pyrrolol2.1-fl [1.2.41triazin-7-yllmethyl}piperazin-2-one (I)
27.1 kg 4-{[4-amino-5-bromo-6-(methoxymethyl)pyrrolo[2,l-f][l,2,4]triazin-7- yl]methyl}piperazin-2-one (VII) were placed in 425 kg THF in a stirred reaction vessel at 10°C jacket temperature. 74 kg of water, 22.1 kg of (7-methoxy-5-methyl-l-benzothiophen- 2-yl)boronic acid (VIII) and 20.5 kg of potassium carbonate were added and the reaction mixture was inerted by reducing internal pressure to 200mbar and refilling to ambient pressure with argon gas. 0.47 kg [l, l'-Bis(di-tert- butylphosphino)ferrocene]dichloropalladium(II) were added and the reactor was inerted by reducing internal pressure to 200mbar and refilling to ambient pressure with argon gas. The reaction mixture was heated to reflux (approx. 64°C internal temperature) within 90 min and stirred for additional 2 h at this temperature. A solution of 24.1 kg acetyl cysteine in 274 kg water was added at 55°C to reflux temperature. The reaction mixture was stirred at this temperature for 2h, then 242 kg of ethyl acetate were added at 55°C to reflux temperature. The jacket temperature was set to 50°C (0355) After stirring for 60 min, the reaction mixture was concentrated by distilling off 366 kg of solvent from the reaction mixture at 48°C to 50°C and 500 mbar. Additional 239 kg of ethyl acetate were added and the reaction mixture was concentrated by distilling off 147 kg of solvent from the reaction mixture at 46°C to 50°C and 500 mbar. After the distillation is finished, the jacket temperature was adjusted to 75°C and the mixture was stirred for 60 min. The mixture was cooled to 20°C internal temperature within 2 h and stirred for additional 2 h. The product was isolated by filtration and washed with a mixture of 196 kg ethanol and 22 kg water. The product is placed in to a stirred reaction vessel in a mixture of 456 kg THF and 91 kg water and heated to 65°C until a solution was obtained. The jacket temperature was set to 50°C and the reaction mixture was concentrated by distillation at 500 to 200 mbar, until no more distillate is collected (approximately 469 kg of distillate). 238 kg of ethanol were added and the reaction mixture was concentrated by distilling off 46 kg of solvent from the reaction mixture at 140 mbar. (0356) The jacket temperature was set to 80°C, the mixture was stirred for 3 h and was then cooled to 15°C internal temperature within 3 h. The mixture was stirred for lh and the product was isolated on a centrifuge and washed with a mixture of 298 kg ethanol and 39 kg water. The product was dried at 45°C and 30 mbar and 28.3 kg of (I) were obtained (0357) This procedure was repeated for producing a second batch of (I) by converting 27.4 kg 4- {[4-amino-5-bromo-6-(methoxymethyl)pyrrolo[2,l-f][l,2,4]triazin-7-yl]methyl}piperazin- 2-one (VII) and 22.1 kg of (7-methoxy-5-methyl-l-benzothiophen-2-yl)boronic acid (VIII) in analogues fashion following method 1 and 28.5 kg of (I) were obtained. Purification: (0359) 28.3 kg of (I) from the first batch of (I) were placed in a stirred reaction vessel in a mixture of 413 kg THF and 81 kg water and heated to reflux at 65°C until a solution was obtained. Then the jacket temperature was set to 60°C and the mixture was passed through a heated particle filter (60°C) into another stirred reaction vessel. (0360) 28.5 kg of (I) from the second batch of (I) were placed in a stirred reaction vessel in a mixture of 410 kg THF and 81 kg water and heated to reflux at 65°C until a solution was obtained. Then the jacket temperature was set to 60°C and the mixture was passed through a heated particle filter (60°C) into the stirred reaction vessel already containing the first portion of the solution of (I). (0361) The jacket temperature was set to 50°C and the reaction mixture was concentrated by distillation at 200 mbar, until no more distillate was collected (approximately 798 kg of distillate). 429 kg of ethanol were added and the reaction mixture was concentrated by distilling off 90 kg of solvent from the reaction mixture at 140 mbar. (0362) The jacket temperature was set to 85°C, the mixture was stirred for 90 min and was then cooled to 15°C internal temperature within 3 h. The mixture was stirred for lh and the product was isolated on a filter dryer and washed with a mixture of 354 kg ethanol and 45 kg water. Finally the product was dried at 45 °C and 30 mbar, yielding 53.6 kg of (I) in 78% of theoretical yield. (0363) HPLC (method 4): (0364) purity: 99.8 % (Rt = 11.6 min ), relevant by-products: (VI) at RRT (relative retention time) of 0.16: n.d.; (XVIII) at RRT 1.02 min: 0.09 %; (XV) at RRT 0.77: n.d. (XVI) at RRT 0.98: n.d. (0365) Assay for use: 97.8 % (against external standard) (0366) Residual solvents (determined by GC method 1): 0.4 % tetrahydrofuran (0367) Residual elements (determined by ICP-MS): 0.9 mg/kg palladium
References:
BAYER AKTIENGESELLSCHAFT;BAYER PHARMA AKTIENGESELLSCHAFT;GRIES, Jörg;PLATZEK, Johannes;HÄSELHOFF, Claus-Christian;LOVIS, Kai WO2020/156982, 2020, A1 Location in patent:Page/Page column 21; 55-58
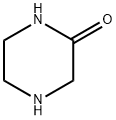
5625-67-2
338 suppliers
$5.00/1g

50-00-0
851 suppliers
$10.00/25g
![Pyrrolo[2,1-f][1,2,4]triazin-4-amine, 6-(methoxymethyl)-5-(7-methoxy-5-methylbenzo[b]thien-2-yl)-](/CAS/20210305/GIF/1443531-77-8.gif)
1443531-77-8
1 suppliers
inquiry

1443530-05-9
67 suppliers
inquiry

83485-34-1
3 suppliers
inquiry

1443530-05-9
67 suppliers
inquiry
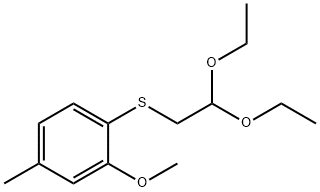
1443531-59-6
5 suppliers
inquiry

1443530-05-9
67 suppliers
inquiry
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)