BAY 87-2243 synthesis
- Product Name:BAY 87-2243
- CAS Number:1227158-85-1
- Molecular formula:C26H26F3N7O2
- Molecular Weight:525.53

27374-25-0
206 suppliers
$13.00/250mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

1227158-85-1
85 suppliers
$65.00/5mg
Yield: 62%
Reaction Conditions:
with acetic acid at 20; for 1.16667 h;Molecular sieve;Reflux;
Steps:
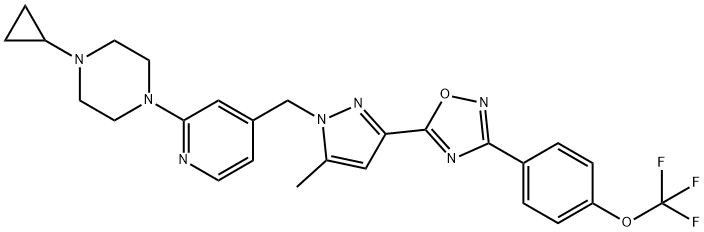
65 1-Cyclopropyl-4-{4-[(5-methyl-3-{3-[4-(trifluoromethoxy)phenyl]-1,2,4-oxadiazol-5-yl}-1H-pyrazol-1-yl)methyl]pyridin-2-yl}piperazine
Example 65
1-Cyclopropyl-4-{4-[(5-methyl-3-{3-[4-(trifluoromethoxy)phenyl]-1,2,4-oxadiazol-5-yl}-1H-pyrazol-1-yl)methyl]pyridin-2-yl}piperazine
66 ml (1.15 mmol) of glacial acetic acid, 13.9 g of dried, powdered molecular sieve (3 Å) and 139 ml (0.692) of 1-ethoxy-1-(trimethylsilyl)oxycyclopropane were added successively to a solution of 56.0 g (0.115 mol) of the compound from Example 64 in 1.13 l of methanol.
After stirring at RT for 10 min, 21.7 g (0.346 mol) of solid sodium cyanoborohydride were added.
The mixture was then heated under reflux for 1 h.
After cooling to RT, the undissolved material was filtered off with suction and the filtrate was concentrated on a rotary evaporator.
The residue obtained was taken up in 1 l of ethyl acetate and the mixture was washed twice with approx.
750 ml of saturated sodium bicarbonate solution each time and then with approx. 750 ml of saturated sodium chloride solution.
After drying over anhydrous sodium sulfate, the mixture was filtered and the filtrate was freed from the solvent on a rotary evaporator.
The residue (53 g) was recrystallized from a boiling mixture of 293 ml of ethanol and 59 ml of water.
When the crystallization was complete (after approx. 20 h at RT), the mixture was filtered with suction.
The solid was washed with 36 ml of ethanol/water (5:1) and then dried under a high vacuum. 26.4 g of the title compound were obtained as the first batch in this way.
The mother liquor of the crystallization was concentrated on a rotary evaporator.
A further 20.3 g of the product were obtained in the form of the formate salt by means of preparative HPLC (method N).
For liberation of the base, a suspension of this formate in 1 l of ethyl acetate was washed successively with approx.
200 ml each of saturated sodium bicarbonate solution, water and saturated sodium chloride solution.
After drying over anhydrous sodium sulfate, the mixture was filtered and the filtrate was freed from the solvent on a rotary evaporator.
The residue (13 g) was recrystallized from a boiling mixture of 80 ml of ethanol and 16 ml of water.
When the crystallization was complete (after approx. 4 h at RT), the mixture was filtered with suction and the solid was dried under a high vacuum.
A further 11.2 g of the title compound were obtained in this manner (yield in total 37.6 g, 62% of th.).
Melting point: 140° C.
1H-NMR (400 MHz, CDCl3, δ/ppm): 8.26 (d, 2H), 8.13 (d, 1H), 7.33 (d, 2H), 6.83 (s, 1H), 6.33 (d, 1H), 6.32 (s, 1H), 5.35 (s, 2H), 3.47 (dd, 4H), 2.69 (dd, 4H), 2.30 (s, 3H), 1.65-1.60 (m, 1H), 0.48-0.42 (m, 4H).
LC/MS (method D, ESIpos): Rt=1.91 min, m/z=526 [M+H]+.
References:
BAYER SCHERING PHARMA AKTIENGESELLSCHAFT;Härter, Michael;Beck, Hartmut;Ellinghaus, Peter;Berhörster, Kerstin;Greschat, Susanne;Thierauch, Karl-Heinz;Süssmeier, Frank US2013/196964, 2013, A1 Location in patent:Paragraph 1599; 1600; 1601; 1602; 1603
![Pyridine, 2-chloro-4-[[5-methyl-3-[3-[4-(trifluoromethoxy)phenyl]-1,2,4-oxadiazol-5-yl]-1H-pyrazol-1-yl]methyl]-](/CAS/20210305/GIF/1225380-68-6.gif)
1225380-68-6
0 suppliers
inquiry

1227158-85-1
85 suppliers
$65.00/5mg