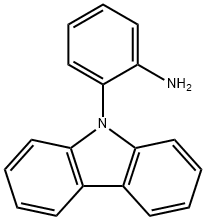
Benzenamine, 2-(9H-carbazol-9-yl)- synthesis
- Product Name:Benzenamine, 2-(9H-carbazol-9-yl)-
- CAS Number:101716-43-2
- Molecular formula:C18H14N2
- Molecular Weight:258.32
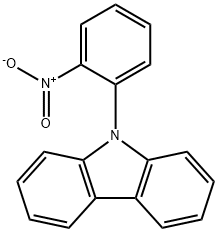
6286-72-2
10 suppliers
$28.60/50MG

101716-43-2
14 suppliers
$376.00/1g
Yield:101716-43-2 89%
Reaction Conditions:
with hydrogenchloride;tin in ethanol;
Steps:
1
Compound A was obtained in a yield of 82% by heat refluxing carbazole and 1-fluoro-2-nitrobenzene in the coexistence of potassium carbonate for 4 hours. The Compound A was reduced with a tin powder and converted into Compound B in a yield of 89%. The Compound B was allowed to react with sodium nitrite in a solvent of sulfuric acid (concentration: 18% by volume) and acetic acid (concentration: 82% by volume) at 0° C. and then condensed by means of heat decomposition, thereby obtaining Compound C in a yield of 62%. The Compound C was brominated in chloroform to obtain Compound D in a yield of 56%. The Compound D was allowed to react with normal butyllithium in a THF solvent. Triphenylchlorosilane was added thereto, and the mixture was allowed to react at room temperature for one hour. The reaction mixture was hydrolyzed with a sodium hydrogencarbonate aqueous solution, thereby obtaining Illustrative Compound 1 in a yield of 27%.1H-NMR data of Illustrative Compound 1: (400 MHz, CDCl3): δ/ppm 8.24 (s, 2H), 8.07 (d, J=7.5 Hz, 2H), 7.92 (d, J=8.05 Hz, 2H), 7.72 to 7.75 (m, 6H), 7.58 to 7.52 (m, 2H), 7.49 to 7.38 (m, 9H), 7.36 to 7.30 (m, 2H)
References:
US2012/178928,2012,A1 Location in patent:Page/Page column 55
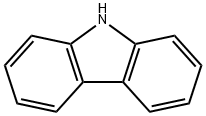
86-74-8
324 suppliers
$10.00/5g
615-36-1
406 suppliers
$10.00/5g

101716-43-2
14 suppliers
$376.00/1g
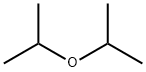
108-20-3
545 suppliers
$25.00/25mL

101716-43-2
14 suppliers
$376.00/1g

86-74-8
324 suppliers
$10.00/5g

101716-43-2
14 suppliers
$376.00/1g

1493-27-2
503 suppliers
$5.00/10g

101716-43-2
14 suppliers
$376.00/1g