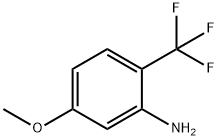
Benzenamine, 5-methoxy-2-(trifluoromethyl)- synthesis
- Product Name:Benzenamine, 5-methoxy-2-(trifluoromethyl)-
- CAS Number:654-83-1
- Molecular formula:C8H8F3NO
- Molecular Weight:191.15
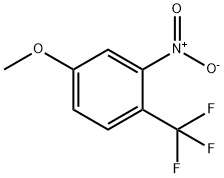
25889-37-6
67 suppliers
$22.00/1g

654-83-1
10 suppliers
inquiry
Yield:654-83-1 96%
Reaction Conditions:
Stage #1: 4-methoxy-2-nitro-1-(trifluoromethyl)benzenewith palladium 10% on activated carbon;hydrogen in ethyl acetate; for 0.25 h;
Stage #2: with palladium(II) hydroxide in ethanol; for 5 h;
Steps:
46 Example 46: Preparation of 5-methoxy-2-(trifluoromethyl)aniline (C247)
A mixture of 4-methoxy-2-nitro-1-(trifluoromethyl)benzene (C285; 0.97 g, 4.4 mmol) and 10% palladium on carbon (0.467 g, 0.44 mmol) in EtOAc (15 mL) was placed on a Parr shaker under hydrogen for 15 min. The reaction was filtered through diatomaceous earth and concentrated to provide a light yellow liquid. The liquid was loaded onto a diatomaceous earth cartridge with DCM. Purification by flash chromatography (0- 50% EtOAc-hexanes) provided the title compound (237 mg). The remaining aniline/nitroso mixture was dissolved in ethanol (15 mL) and catalytic palladium hydroxide was added under a nitrogen atmosphere. The reaction mixture was placed on Parr shaker for 5 h. The reaction mixture was filtered through diatomaceous earth, the cake was rinsed with EtOAc, and the filtrate concentrated. The title compound was isolated as a light yellow liquid (819 mg, 96%):1H NMR (400 MHz, CDCl3) d 7.34 (d, J = 8.8 Hz, 1H), 6.33 (ddt, J = 8.8, 2.4, 0.8 Hz, 1H), 6.27- 6.17 (m, 1H), 4.19- 4.09 (m, 2H), 3.79 (s, 3H);19F NMR (376 MHz, CDCl3) d -61.25; EIMS m/z 191.
References:
WO2021/11722,2021,A1 Location in patent:Page/Page column 212-213
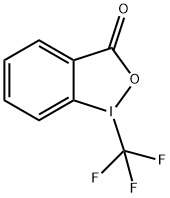
887144-94-7
176 suppliers
$19.00/1g

536-90-3
292 suppliers
$10.00/5g

654-83-1
10 suppliers
inquiry

106877-20-7
66 suppliers
$37.00/250mg
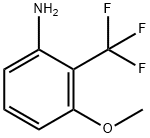
53982-03-9
16 suppliers
$64.09/250mg
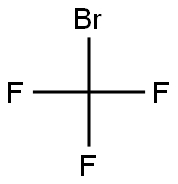
75-63-8
1 suppliers
inquiry

536-90-3
292 suppliers
$10.00/5g

654-83-1
10 suppliers
inquiry

106877-20-7
66 suppliers
$37.00/250mg

53982-03-9
16 suppliers
$64.09/250mg
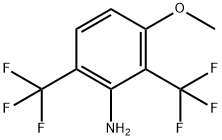
106877-19-4
2 suppliers
inquiry