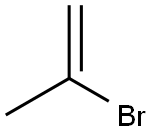
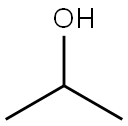
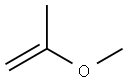
Benzenamine, N-(1-methylethylidene)- synthesis
- Product Name:Benzenamine, N-(1-methylethylidene)-
- CAS Number:1124-52-3
- Molecular formula:C9H11N
- Molecular Weight:133.19
Yield:1124-52-3 97%
Reaction Conditions:
with microporous zeolite at 230; for 24 h;Sealed tube;Autoclave;
Steps:
Synthesis of 2,2,4-trimethyl-1,2-dihydroquinoline (1) and N-phenyl-2-propanimine (2).
General procedure: Aniline (0.1 g, 1 mmol), acetone (0.3 g, 5 mmol), and a catalyst (0.04 g, 10% calculated on the aniline-acetone mixture) were placed into a tube. The sealed tube was transferred into an autoclave, which was heated to 230 °C in a thermostatically controlled oven during 24 h with continuous rotation. After the reaction reached completion, the autoclave was cooled to ~20 °C, the tube was unsealed. The reaction mixture was filtered from the catalyst
References:
Grigor’eva;Filippova;Gataulin;Bubennov;Agliullin;Kutepov;Narender, Nama [Russian Chemical Bulletin,2017,vol. 66,# 11,p. 2115 - 2121][Izv. Akad. Nauk, Ser. Khim.,2017,# 11,p. 2115 - 2121,7]

62-53-3
651 suppliers
$10.00/1g

67-64-1
6 suppliers
$19.10/10ml

1124-52-3
0 suppliers
inquiry
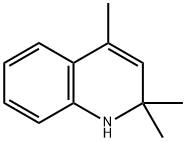
147-47-7
91 suppliers
$7.00/250mg