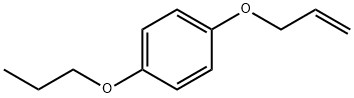
Benzene, 1-(2-propen-1-yloxy)-4-propoxy- synthesis
- Product Name:Benzene, 1-(2-propen-1-yloxy)-4-propoxy-
- CAS Number:1005006-02-9
- Molecular formula:C12H16O2
- Molecular Weight:192.25
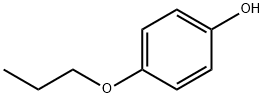
18979-50-5
113 suppliers
$8.00/250mg

106-95-6
418 suppliers
$10.00/5g

1005006-02-9
0 suppliers
inquiry
Yield: 69%
Reaction Conditions:
with potassium carbonate in acetoneReflux;
Steps:
Aryl allyl ethers (2-2g). General procedure[11].
General procedure: A slurry of 6 mmol of an appropriate phenol 3-3g, 0.8 g (6.6 mmol) of allyl bromide, and 0.91 g (6.6 mmol) of crushed calcined K2CO3 in 10 mL of anhydrous acetone was boiled at stirring for 10-12 h. The reaction was monitored by TLC (Rf of reaction products ~0.7, eluent hexane-EtOAc, 9 : 1). Then the reaction mixture was diluted with water (30 mL), the reaction products were extracted with Et2O (3 × 40 mL). The combined extracts were washed with 1 M water solution of NaOH (15 mL) and dried with MgSO4. The solvent was removed at a reduced pressure. The obtained lowmelting crystals 2b, 2c, and 2g or oily substances 2and 2d-2f were purified by column chromatography on silica gel, eluent hexane-EtOAc, 9 : 1-8 : 2.
References:
Fayzullin;Antonovich;Zakharychev;Bredikhina;Kurenkov;Bredikhin [Russian Journal of Organic Chemistry,2015,vol. 51,# 2,p. 202 - 209][Zh. Org. Khim.,2015,vol. 51,# 2,p. 214 - 221]
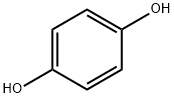
123-31-9
826 suppliers
$13.00/25g

1005006-02-9
0 suppliers
inquiry