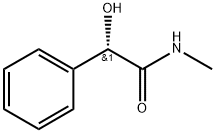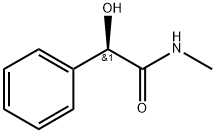
Benzeneacetamide, α-hydroxy-N-methyl-, (R)- (9CI) synthesis
- Product Name:Benzeneacetamide, α-hydroxy-N-methyl-, (R)- (9CI)
- CAS Number:89843-35-6
- Molecular formula:C9H11NO2
- Molecular Weight:165.19
Yield:68 % ee
Reaction Conditions:
with alpha-D-glucopyranose;Candida parapsilosis ATCC 7330 in acetonitrile at 25; pH=7.2; for 24 h;Enzymatic reaction;
Steps:
2.7 Biocatalytic reduction of secondary -keto amides 1a1j using whole cells of C. parapsilosis ATCC 7330
General procedure: 6.4 g wet cells of C. parapsilosis ATCC 7330 was suspended in 16 ml of 10 mM potassium phosphate buffer (pH 7.2). 5 mmol glucose and 10 mg of 1a (0.042 mmol) dissolved in 0.5 ml acetonitrile were added into the cell suspension; incubated in a shaker at 200 rpm and 25 °C for 24 h. The reaction was done in triplicates. The product R-2a was extracted by ethylacetate (thrice with 10 ml) and concentrated under high vacuum. The crude product was purified by column chromatography by hexane: ethylacetate mixture (4:1) to give 71% yield of R-2a. The ee of R-2a (68%) was determined by chiral HPLC OJ-H column using 9:1 hexane:isopropanol mixture. The specific rotation of R-2a {[]D23 = 30.5 (c 1, CHCl3) for 68% ee} was compared with the literature value {Lit. []D25 = 82.4 (c 1.09, CHCl3) for >99% ee [5]} to assign the absolute configuration. It was found to be R. The same procedure was followed for the reduction of remaining secondary -keto amides. The product R-2j is slightly water soluble and that accounts for the reduced isolated yield as compared to its conversion (Table 2, entry 5). The products R-2b [5], R-2c [41], R-2d [54], R-2e and R-2f were not isolated due to their low conversion/ee.
References:
Stella, Selvaraj;Chadha, Anju [Catalysis Today,2012,vol. 198,# 1,p. 345 - 352]