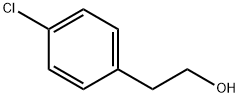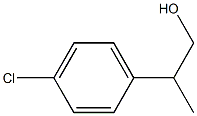
Benzeneethanol, 4-chloro-b-methyl- synthesis
- Product Name:Benzeneethanol, 4-chloro-b-methyl-
- CAS Number:59667-21-9
- Molecular formula:C9H11ClO
- Molecular Weight:170.636
Yield:59667-21-9 98%
Reaction Conditions:
with C24H24IrN4O4(1+)*BF4(1-);sodium t-butanolate at 140; for 24 h;Sealed tube;Inert atmosphere;
Steps:
General procedure for bis-NHC-Ir-catalyzed β-methylationof primary alcohols
General procedure: To a sealed tube (35 mL) equipped witha stir bar, Ir-NHC catalyst 5d (0.05 mol%), methanol (1 mL),tBuONa (2 mmol) and primary alcohol (1 mmol) were addedunder nitrogen atmosphere. The solution was heated at140 °C for 24 h. 1,3,5-Trimethoxybenzene was added as aninternal standard, and sent for nuclear magnetic resonance(NMR) measurement. Pure products were obtained by columnchromatography over silica gel using ethyl acetate/petroleum ether mixture as eluent.
References:
Lu, Zeye;Zheng, Qingshu;Zeng, Guangkuo;Kuang, Yunyan;Clark, James H.;Tu, Tao [Science China Chemistry,2021,vol. 64,# 8,p. 1361 - 1366]

637-87-6
288 suppliers
$38.00/10g

107-18-6
2 suppliers
$24.60/100ml
6282-88-8
156 suppliers
$16.00/1g

59667-21-9
2 suppliers
inquiry
938-95-4
86 suppliers
$31.80/1gm:

59667-21-9
2 suppliers
inquiry