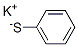
Benzenethiol, potassium salt synthesis
- Product Name:Benzenethiol, potassium salt
- CAS Number:3111-52-2
- Molecular formula:C6H5S.K
- Molecular Weight:0
882-33-7
312 suppliers
$10.00/5g
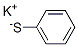
3111-52-2
27 suppliers
$198.00/10g
Yield:-
Reaction Conditions:
with hydrazine hydrate;potassium hydroxide at 85 - 90; for 2.5 h;
Steps:
2,3-Bis(phenylsulfanyl)prop-1-ene (7b). A.
A mixture of KOH (3.21 g, 0.057 mol), hydrazine hydrate (14 mL), Ph2S2 (2.5 g,11.4 mmol), and dichloropropene 1 (1.27 g, 11.4 mmol) was heated for 2 h (60 „aC). The yield of the product was 2%. A workup gave 1.48 g of the residue (a light brown liquid) containing (1H NMR data) a mixture of compounds 4b, 5b, Z6b, E6b, and7b in the ratio 1.0 : 41.0 : 25.5 : 1.4 : 2.35. 1H NMR of compound7b, : 3.61 (d, 2 H, CH2S, 4JHH = 1.2 Hz); 5.04 (s, 1 H, cisCH=);5.34 (t, 1 H, transCH=, 4JHH = 1.2 Hz); 7.10X7.40 (m, 10 H, Ph).B. The compound Ph2S2 (1.0 g, 4.6 mmol) was added to a solution of KOH (1.3 g, 0.023 mol) in hydrazine hydrate (6 mL). The reaction mixture was stirred for 2.5 h at 85-90 °C and cooled to 60° C, followed by a dropwise addition of compound 3b (1.69 g, 9.2 mmol) and stirring for 5 h at this temperature. A workup gave a light yellow liquid (1.55 g), which was a mixture of compounds 4b, 5b, Z-6b, E-6b, and 7b in the ratio 1.0 : 47.2 : 57.5 : 3.0 : 7.0. The mixture contained compound 7b (0.115 g, 5%).
References:
Levanova;Vakhrina;Grabel'Nykh;Rozentsveig;Russavskaya;Albanov;Korchevin [Russian Chemical Bulletin,2014,vol. 63,# 8,p. 1722 - 1727][Izvestiya Akademii Nauk. Seriya Khimicheskaya,2014,# 8,p. 1722 - 1727,6]
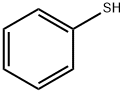
108-98-5
0 suppliers
$21.10/10g
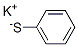
3111-52-2
27 suppliers
$198.00/10g

![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)