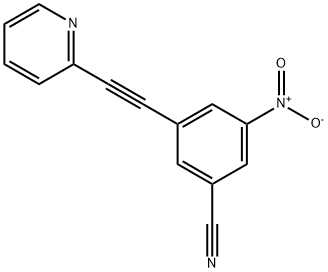
Benzonitrile, 3-nitro-5-[2-(2-pyridinyl)ethynyl]- synthesis
- Product Name:Benzonitrile, 3-nitro-5-[2-(2-pyridinyl)ethynyl]-
- CAS Number:1031370-96-3
- Molecular formula:C14H7N3O2
- Molecular Weight:249.22
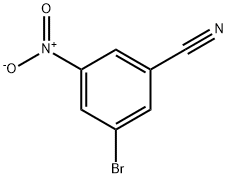
49674-15-9
77 suppliers
inquiry
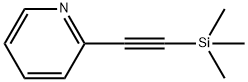
86521-05-3
90 suppliers
$11.00/250mg
![Benzonitrile, 3-nitro-5-[2-(2-pyridinyl)ethynyl]-](/CAS/20210111/GIF/1031370-96-3.gif)
1031370-96-3
6 suppliers
inquiry
Yield:1031370-96-3 61%
Reaction Conditions:
Stage #1: 3-bromo-5-nitrobenzonitrile;2-(trimethylsilylethynyl)pyridinewith bis-triphenylphosphine-palladium(II) chloride;copper(l) iodide;triethylamine in N,N-dimethyl-formamide at 60;Sonogashira coupling;
Stage #2: with tetrabutyl ammonium fluoride in tetrahydrofuran;N,N-dimethyl-formamide at 60; for 2 h;
Steps:
3-(2-(pyridin-2-yl)ethynyl)-5-nitrobenzonitrile 4a
General procedure: To a solution of 8 (500 mg, 2.85 mmol) in 10 mL of DMF was successively added Et3N (1.59 mL, 11.42 mmol), 9a (647 mg, 2.86 mmol), CuI (53 mg, 0.28 mmol), and trans-dichlorobis(triphenylphosphine)palladium (198 mg, 0.28 mmol). The resulting solution was warmed to 60 oC, and TBAF 1M in THF (3.13 ml, 3.13mmol) was added dropwise. After 2h at this temperature the mixture was hydrolyzed with 50 mL of H2O and, extracted with AcOEt (4 X 30 mL). The organic layer was washed with saturated NaCl (3 X 30 mL), dry over anhydrous Na2SO4, and concentrated on a rotary evaporator. Purification of the residue by column chromatography (hexane/AcOEt 8/2) yielded 434 mg (61 %) of 4a as an off white solid.1H NMR (CDCl3), d = 7.26-7.29 (m, 1H, CHAr); 7.51 (dd, 1H, J=7.6, 1.2 Hz, CHAr); 7.69 (td, 1H, J=7.6, 1.6 Hz, CHAr); 8.06 (t, 1H, J=1.2 Hz, CHAr); 8.39 (t, 1H, J=1.2 Hz, CHAr); 8.53 (t, 1H, J=1.2 Hz, CHAr); 8.59 (dd, 1H, J=4.8, 0.8 Hz, CHAr). 13C NMR (CDCl3), d = 83.9 (1C, C≡C); 93.3 (1C, C≡C); 114.5 (1C, Cq); 115.9 (1C, Cq); 124.1 (1C, CHAr); 126.0 (1C, Cq); 126.6 (1C, CHAr); 126.7 (1C, CHAr); 127.7 (1C, CHAr); 130.4 (1C, CHAr); 136.5 (1C, CHAr); 140.1 (1C, CHAr); 141.6 (1C, Cq); 148.2 (1C, Cq); 150.5 (1C, CHAr). Anal (C14H7N3O2, HCl) C, H, N.
References:
Alagille, David;Dacosta, Herve;Chen, Yelin;Hemstapat, Kamondanai;Rodriguez, Alice;Baldwin, Ronald M.;Conn, Jeffrey P.;Tamagnan, Gilles D. [Bioorganic and Medicinal Chemistry Letters,2011,vol. 21,# 11,p. 3243 - 3247] Location in patent:supporting information; experimental part
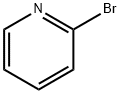
109-04-6
523 suppliers
$6.00/25g
![Benzonitrile, 3-nitro-5-[2-(2-pyridinyl)ethynyl]-](/CAS/20210111/GIF/1031370-96-3.gif)
1031370-96-3
6 suppliers
inquiry
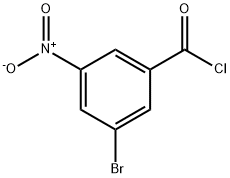
51339-40-3
8 suppliers
inquiry
![Benzonitrile, 3-nitro-5-[2-(2-pyridinyl)ethynyl]-](/CAS/20210111/GIF/1031370-96-3.gif)
1031370-96-3
6 suppliers
inquiry
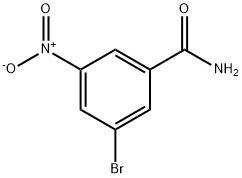
54321-80-1
42 suppliers
$55.00/250mg
![Benzonitrile, 3-nitro-5-[2-(2-pyridinyl)ethynyl]-](/CAS/20210111/GIF/1031370-96-3.gif)
1031370-96-3
6 suppliers
inquiry
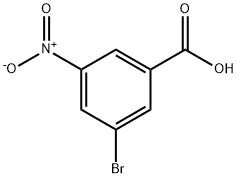
6307-83-1
210 suppliers
$5.00/250mg
![Benzonitrile, 3-nitro-5-[2-(2-pyridinyl)ethynyl]-](/CAS/20210111/GIF/1031370-96-3.gif)
1031370-96-3
6 suppliers
inquiry