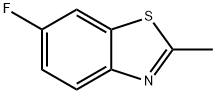
Benzothiazole, 6-fluoro-2-methyl- (7CI,8CI,9CI) synthesis
- Product Name:Benzothiazole, 6-fluoro-2-methyl- (7CI,8CI,9CI)
- CAS Number:399-73-5
- Molecular formula:C8H6FNS
- Molecular Weight:167.2
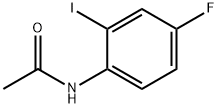
29654-01-1
0 suppliers
inquiry

399-73-5
57 suppliers
$51.00/250mg
Yield:399-73-5 77%
Reaction Conditions:
Stage #1: N-(2-iodo-4-fluorophenyl)acetamidewith sodiumsulfide nonahydrate in N,N-dimethyl-formamide at 80; for 12 h;Schlenk technique;Sealed tube;
Stage #2: with hydrogenchloride in N,N-dimethyl-formamide at 20; for 10 h;
Steps:
General Procedure for Synthesis of Substituted Benzothiazoles Catalyzedby MCM-41-NHC-CuI
General procedure: To an oven-dried Schlenk tube were added Na2S?9H2O (or K2S, 3.0mmol), o-haloanilide (1.0 mmol), and MCM-41-NHC-CuI (228 mg, 0.1 mmol). The tube was sealed and then evacuated and backfilled withargon, and DMF (2 mL) was injected with a syringe. The mixture washeated to 80 C with stirring for 12 h (140 C and 24 h for o-bromoanilide).Upon cooling to ambient temperature, the mixture was centrifugatedto separate the copper catalyst. The catalyst recovered waswashed with deionized water (3 × 2 mL) and acetone (3 × 2 mL) anddried in vacuo at 80 C for 1 h, and used in the next run. Then conc. HCl(0.8 mL) was added into the resultant solution. After stirring for 10 h atambient temperature, 10 mL saturated aq. NaHCO3 was added into thesolution and the resulting mixture was then extracted with EtOAc forthree times. After being washed with water and brine, the organic layerwas dried over anhydrous MgSO4 and concentrated under the reducedpressure. The residue was then purified via column chromatography onsilica gel (hexane/ethyl acetate) to furnish the expected product 2.
References:
Cai, Mingzhong;Hao, Wenyan;Huang, Wencheng;Ye, Qian [Molecular catalysis,2022,vol. 519,art. no. 112115]
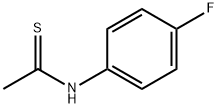
351-84-8
6 suppliers
inquiry

399-73-5
57 suppliers
$51.00/250mg
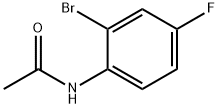
1009-22-9
221 suppliers
$17.80/5g

399-73-5
57 suppliers
$51.00/250mg
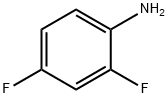
367-25-9
488 suppliers
$5.00/5g

399-73-5
57 suppliers
$51.00/250mg
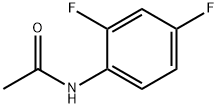
399-36-0
82 suppliers
$20.00/5g

399-73-5
57 suppliers
$51.00/250mg